- Cancer Immunotherapy and How It Works
- Releasing the Brakes on the Immune System
- Boosting the Cancer-killing Power of Immune Cells
- Directing the Immune System to Cancer Cells
- Current Challenges in Cancer Immunotherapy
- Knowledge Gaps in Predicting Response to Immunotherapy
- Adverse Effects of Immunotherapy
- Disparities in Access to Immunotherapy
- On the Horizon for Immunotherapy
Spotlight on Immunotherapy: Pushing the Frontier of Cancer Medicine
In this section, you will learn:
- Cancer immunotherapeutics work by unleashing the power of a patient’s immune system to fight cancer and, over the last decade, have emerged as one of the most exciting new approaches to cancer treatment.
- Immune checkpoint inhibitors (ICIs) work by releasing the brakes on the natural cancer-fighting power of the immune system. As of July 31, 2023, the FDA has approved 11 ICIs, and there is at least one ICI approved for treating 20 cancer types and for treating any type of solid tumors that share certain molecular characteristics.
- CAR T-cell therapy provides more cancer-targeted immune cells called T cells. As of July 31, 2023, the FDA has approved six distinct CAR T-cell therapies for the treatment of a range of hematologic cancers.
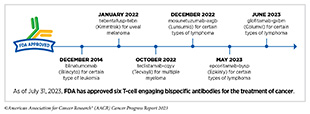
- T-cell engaging bispecific antibodies work by flagging cancer cells for destruction by the immune system. As of July 31, 2023, the FDA has approved six different T-cell engaging bispecific antibodies for the treatment of many cancers, including some extremely rare cancers.
- Treatment with immunotherapeutics can lead to severe adverse effects and ongoing research is evaluating biomarkers that can identify patients who are most likely to respond to these treatments.
- The new frontier of immunotherapy, which includes preventive and therapeutic vaccines, innovative cell therapies, novel checkpoint inhibitors, and a new age of treatment combinations, is poised to transform the future of clinical cancer care.
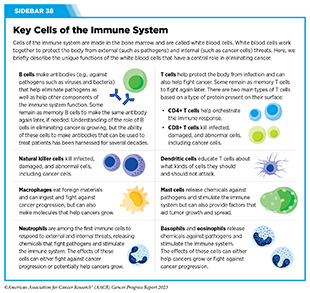
The immune system is a complex network of cells (called white blood cells; see Sidebar 38), tissues (e.g., bone marrow), organs (e.g., thymus), and the substances they make that help the body fight infections and other diseases, including cancer. The immune system actively detects threats from external (such as viruses and bacteria) and internal sources (such as abnormal or damaged cells) and works to eliminate them from the body.
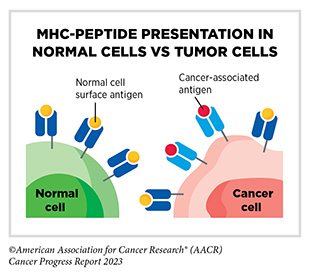
The immune system is highly effective in detecting and eliminating cancer cells, a process also known as cancer immune surveillance (417)Hiam-Galvez KJ, et al. (2021) Nat Rev Cancer, 21: 345. [LINK NOT AVAILABLE]. However, as cancer cells acquire new properties during the course of disease progression (see Understanding the Path to Cancer Development), some cells find ways to “hide” from the immune system, such as by decreasing or eliminating the numbers and/or amounts of proteins on the surface of tumor cells that are used by the immune system to recognize cancer cells; triggering certain brakes on immune cells that prevent them from eradicating cancer cells; and releasing molecules that weaken the ability of immune cells to detect and destroy cancer cells (418)Mishra AK, et al. (2022) Diseases, 10. [LINK NOT AVAILABLE]. Ongoing research is focused on better understanding how tumor cells evade the immune system and leveraging this knowledge to develop additional effective cancer treatments.
Cancer Immunotherapy and How It Works
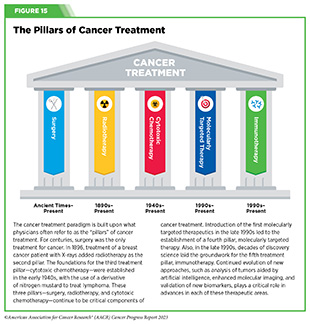
Cancer immunotherapy refers to any treatment that works by using the immune system to fight cancer. The history of invoking the immune system to treat cancer dates back to the late 19th century, when William B. Coley, a surgeon in Memorial Hospital, New York, injected bacteria into patients with inoperable malignant cancers based on his observation that an infection appeared to have the side effect of shrinking tumors. He saw excellent responses in a fraction of more than 1,000 patients with cancer he treated, and these bacterial products became known as Coley’s toxins (419)McCarthy EF (2006) Iowa Orthop J, 26: 154. [LINK NOT AVAILABLE]. The use of Coley’s toxins was discontinued because of the risk of severe side effects, the emergence of radiotherapy and chemotherapy, and because scientists did not have the ability at that time to fully understand the mechanism by which they worked or failed. Over the past five decades, researchers have made unprecedented advances in understanding how the immune system detects and destroys cancer cells in the human body, which has reinvigorated the field of cancer immunology and has firmly established immunotherapy as the fifth pillar of cancer medicine (420)Kaufmann SHE (2019) Front Immunol, 10: 684. [LINK NOT AVAILABLE].
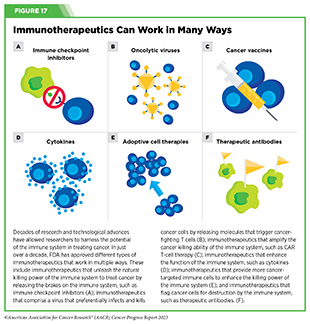
Groundbreaking basic research in the field of immunology— the study of the immune system—has laid the foundation of modern immunotherapy, one of the most exciting new areas of cancer treatment along with molecularly targeted therapeutics (see Figure 15). Cancer immunotherapeutics leverage the natural ability of the immune system to fight cancer (see Figure 17) (421)Waldman AD, et al. (2020) Nat Rev Immunol, 20: 651. [LINK NOT AVAILABLE]. There are various ways in which different immunotherapeutics unleash the immune system to fight cancer.
Some immunotherapeutics work by unleashing the natural cancer-fighting powers of the immune system. Immune checkpoint inhibitors (ICIs) are examples of immunotherapeutics that work in this way. ICIs have revolutionized the landscape of cancer treatment and, over the past decade, have been approved widely by FDA for use in the treatment of diverse cancer types (see Releasing the Brakes on the Immune System). Some immunotherapeutics dramatically amplify the cancer killing power of the immune system by either providing more cancer-targeted immune cells (see Adoptive Cell Therapy), or by enhancing T-cell function utilizing proteins known as cytokines (see Enhancing Immune Cell Function) or cells that trigger cancer-fighting T cells (e.g., the cancer vaccine sipuleucel-T or Provenge).
Other immunotherapeutics work by flagging cancer cells for rapid destruction by the immune system. These include therapeutic antibodies that work by bringing immune cells into close contact with cancer cells and activating them, which leads to cancer cell destruction (422)Goebeler ME, et al. (2020) Nat Rev Clin Oncol, 17: 418. [LINK NOT AVAILABLE] (see Directing the Immune System to Cancer Cells).
Yet other immunotherapeutics comprise a virus that preferentially infects and kills cancer cells, releasing molecules that trigger cancer-fighting T cells; these are called oncolytic virotherapeutics, for example, talimogene laherparepvec (T-Vec; Imlygic).
Releasing the Brakes on the Immune System
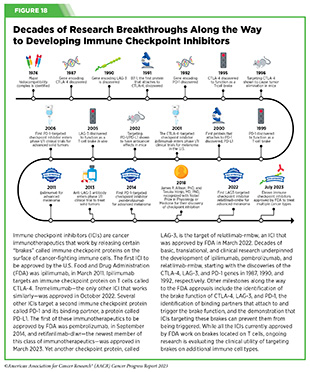
Decades of research have revealed that some tumor cells have increased levels of certain proteins on their surface that attach to and activate “brakes” on T cells, thus stopping them from attacking cancer cells. These brakes are proteins on the surface of T cells and are called immune checkpoint proteins. Immune checkpoint inhibitors (ICIs) are a class of transformative new therapeutics that can release the brakes on T cells and trigger T cells to destroy cancer cells (423)Marin-Acevedo JA, et al. (2021) J Hematol Oncol, 14: 45. [LINK NOT AVAILABLE].
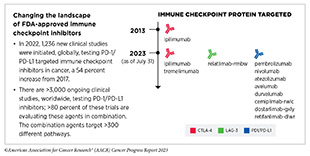
The story of ICIs began in 1987, when researchers discovered a gene that they called CTLA-4 (see Figure 18) (424)Pardoll DM (2012) Nat Immunol, 13: 1129. [LINK NOT AVAILABLE]. However, it took nearly eight years before the immune checkpoint function of CTLA-4 was discovered, and another 16 years of basic and clinical research before this knowledge was translated into a clinically effective therapy, a therapeutic antibody that targets CTLA-4, ipilimumab (Yervoy). Upon attaching to CTLA-4 on the surface of patients’ T cells, ipilimumab releases a set of brakes on T cells, spurring them into action. Ipilimumab was the first treatment in history to improve survival for patients with metastatic melanoma and was approved by FDA for this use in March 2011.
Motivated by the success of ipilimumab and the need to provide new treatment options for patients who did not achieve long-term response to ipilimumab, researchers focused on targeting a second checkpoint protein, PD-1, as well as one of the proteins that attaches to it, PD-L1. The first FDA approval of an ICI targeting PD-1 or PD-L1 occurred in September 2014, when pembrolizumab (Keytruda), which targets PD-1, was approved for treating certain patients with metastatic melanoma. Notably, since the approval of pembrolizumab, three additional ICIs have been approved by FDA for the treatment of patients with metastatic melanoma, to be used alone or in combination with another ICI or molecularly targeted therapeutics. Thanks to these clinical breakthroughs, mortality from melanoma has declined significantly, for the first time in four decades, during the period between 2013 and 2017, and continues to trend downward (425)Kahlon N, et al. (2022) JAMA Netw Open, 5: e2245269. [LINK NOT AVAILABLE].
The third immune checkpoint protein targeted by an ICI is LAG-3. The first and, so far, only LAG-3 targeted ICI, relatlimab-rmbw, was approved by FDA in March 2022 in combination with nivolumab (Opdualag) for treatment of certain patients with melanoma.
Two researchers whose pioneering work established the field of ICIs, James P. Allison, PhD, and Tasuku Honjo, MD, PhD, were recognized with the 2018 Nobel Prize in Physiology or Medicine for “their discovery of cancer therapy by inhibition of negative immune regulation.”
The use of ICIs in the treatment of cancer has rapidly expanded over the last decade and these therapeutics are considered one of the most exciting approaches to cancer treatment. This is in part because some patients with metastatic disease who have been treated with these therapeutics have had remarkable and durable responses. As one example, long-term results from a clinical trial testing the ICI pembrolizumab for patients with advanced NSCLC showed that 23 percent lived 5 or more years, which stands in stark contrast to the historical 5-year relative survival rate for patients with advanced NSCLC of about 5 percent (426)Garon EB, et al. (2019) J Clin Oncol, 37: 2518. [LINK NOT AVAILABLE]. Recent analysis suggests that the use of ICIs is also favorably associated with patients’ quality of life (427)Pala L, et al. (2022) JAMA Netw Open, 5: e2226252. [LINK NOT AVAILABLE].
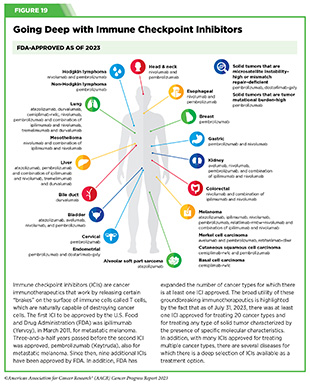
As of July 31, 2023, FDA has approved eleven ICIs, targeting one of three different T-cell brakes, CTLA-4, PD-1/PD-L1, or LAG-3. Notably, these groundbreaking treatments are FDA approved for an increasingly broad array of cancer types (see Figure 19). As of July 31, 2023, there was at least one checkpoint inhibitor approved for treating 20 cancer types and for treating any type of solid tumor characterized by the presence of either of three specific molecular characteristics (see Figure 19).
A watershed moment in cancer medicine was the May 2017 FDA approval of the ICI pembrolizumab for treatment of patients with solid tumors not of a specific organ/tissue type but rather characterized by the presence of either of two specific molecular characteristics, or biomarkers, called microsatellite instability– high and DNA mismatch–repair deficiency. These biomarkers are found in a small proportion of cancers arising at numerous sites in the body, including the colon, endometrium, stomach, and rectum. This was the first ever approval of a therapeutic to treat cancer based solely on its molecular alterations rather than the site of origin. It was also an example of precision immunotherapy, whereby a patient’s immunotherapy is tailored to the molecular characteristics of his or her tumor. Remarkable progress in our understanding of cancer biology and in the conduct of clinical research led to this approval. Since 2017, pembrolizumab has been approved for the treatment of patients with tumors characterized by another biomarker known as high tumor mutational burden (TMB); additionally, a second ICI, dostarlimab-gxly, and several molecularly targeted therapeutics have received site-agnostic FDA approvals based on biomarkers and are providing new treatment options and new hope to patients with a wide range of cancer types.
During the 12 months spanning this report, August 1, 2022, to July 31, 2023, FDA approved two new ICIs for the first time— tremelimumab (Imjudo) and retifanlimab-dlwr (Zynyz)—and expanded the uses of two of the previously approved ICIs— durvalumab (Imfinzi) and atezolizumab (Tecentriq)—to treat additional types of cancer.
In October 2022, tremelimumab (Imjudo) became the tenth ICI to be approved by FDA. It was approved in combination with another ICI, durvalumab (Imfinzi), for treating adult patients with hepatocellular carcinoma (HCC, a type of liver cancer) whose tumor is not removable with surgery. Tremelimumab is the second FDA-approved ICI that targets the checkpoint protein CTLA-4, while durvalumab blocks the checkpoint protein PD-L1. Combined targeting of the two checkpoints, CTLA-4 and PD-1/PD-L1, has shown clinical benefit in a number of cancer types.
HCC is the most commonly diagnosed liver cancer in adults and the third leading cause of cancer-related deaths worldwide (428)Zhuo Y, et al. Changing Epidemiology of Hepatocellular Carcinoma and Role of Surveillance. In: Hoshida Y, editor. Hepatocellular Carcinoma: Translational Precision Medicine Approaches. Cham (CH)2019. p 53. [LINK NOT AVAILABLE]. Most HCC cases are diagnosed at an advanced stage when surgical removal is not an option, and many patients experience cancer recurrence after initial treatment (429)Abou-Alfa GK, et al. (2022) NEJM Evidence, 1: EVIDoa2100070. [LINK NOT AVAILABLE]. Patients with HCC that is not removable surgically are treated with systemic therapy, including anti-angiogenic treatments that block the development of blood vessels or with ICIs, often as combination therapy. While these treatments have led to some improvement in survival for patients with HCC not surgically removable, additional treatment options are needed for this globally diverse patient population, highlighting the importance of FDA approval of the tremelimumab and durvalumab combination.
The efficacy of tremelimumab plus durvalumab was tested in a randomized, phase III clinical trial in patients with unresectable HCC who had not received prior systemic treatment. The study compared the efficacy of tremelimumab plus durvalumab to the standard of care, which is treatment with the molecularly targeted antiangiogenic therapeutic sorafenib (Nexavar). Findings from the trial showed that patients who received the ICI combination experienced a 22 percent reduction in the risk of death during the course of the study compared to patients who received sorafenib (429)Abou-Alfa GK, et al. (2022) NEJM Evidence, 1: EVIDoa2100070. [LINK NOT AVAILABLE].
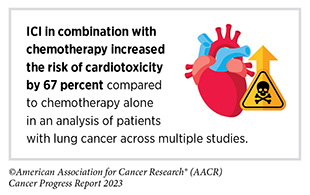
In the 12 months covered in this report, FDA also expanded the approval of the tremelimumab and durvalumab combination for certain patients with lung cancer. In November 2022, FDA approved tremelimumab in combination with durvalumab and platinum-based chemotherapy for treatment of adult patients with metastatic NSCLC whose tumors do not have mutations in the EGFR or ALK genes. Patients with NSCLC with tumors that have alterations in EGFR or ALK are usually treated with molecularly targeted therapeutics that specifically target the EGFR or ALK proteins.
The approval was based on results from a phase III randomized clinical trial that compared the efficacy of the tremelimumab, durvalumab, and chemotherapy combination to chemotherapy alone, among patients with NSCLC who had not received prior systemic treatment. The data from the clinical trial showed that patients receiving the ICI combination experienced a 23 percent reduction in the risk of death during the course of the trial compared to those receiving chemotherapy alone (430)Johnson ML, et al. (2023) J Clin Oncol, 41: 1213. [LINK NOT AVAILABLE]. The median overall survival (see Sidebar 32) was 14 months in patients treated with the combination versus 11.7 months in patients treated with chemotherapy alone (430)Johnson ML, et al. (2023) J Clin Oncol, 41: 1213. [LINK NOT AVAILABLE].
In September 2022, FDA approved durvalumab in combination with the chemotherapeutics gemcitabine and cisplatin for adult patients with locally advanced or metastatic cancer of the biliary tract.
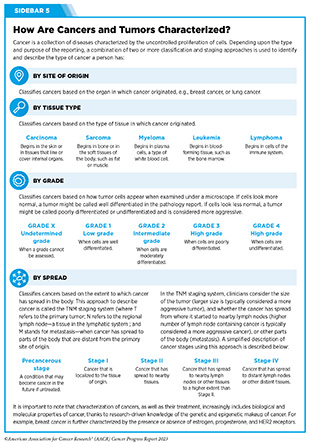
Biliary tract cancers include various rare but aggressive epithelial malignancies (see Sidebar 5), including bile duct cancer and gallbladder cancer, among others and account for three percent of all gastrointestinal cancers. Surgical removal of the entire tumor is currently the only curative treatment for biliary tract cancer. Unfortunately, many patients are diagnosed at an advanced stage when surgery is no longer an option. For over a decade, the combination of gemcitabine and cisplatin has remained the standard initial treatment for patients with advanced biliary tract cancer, including those whose cancer returned after the initial treatment.
Research has shown that the tumor microenvironment of biliary tract cancers may suppress the immune system, indicating that there may be limited benefit from immunotherapeutics, if used alone. Chemotherapeutics are known to modulate the immune system, leading researchers to hypothesize that a combination of ICIs with chemotherapy could improve response to immunotherapy. Recent data from two clinical trials supported this hypothesis and showed that patients with advanced biliary tract cancer benefit when ICIs such as durvalumab or pembrolizumab are administered as a combination with gemcitabine and cisplatin (431)Oh D-Y, et al. (2022) NEJM Evidence, 1: EVIDoa2200015. [LINK NOT AVAILABLE](432)Kelley RK, et al. (2023) Lancet, 401: 1853. [LINK NOT AVAILABLE]. One of these trials provided the basis for the approval of durvalumab in biliary tract cancer.
The study, a phase III randomized clinical trial, found that adding durvalumab to standard chemotherapy modestly extended how long patients with advanced biliary tract cancer lived (431)Oh D-Y, et al. (2022) NEJM Evidence, 1: EVIDoa2200015. [LINK NOT AVAILABLE]. The FDA approval was based on the fact that patients who received chemotherapy plus durvalumab had a 20 percent reduction in the risk of death during the course of the trial compared to patients who received chemotherapy alone (431)Oh D-Y, et al. (2022) NEJM Evidence, 1: EVIDoa2200015. [LINK NOT AVAILABLE]. While these data offer hope for patients with a very aggressive disease, there is urgent need for continued research to identify more effective treatments for this difficult-to-treat group of cancers.
In addition to combinations with other ICIs, researchers are combining ICIs with molecularly targeted therapeutics to maximize the potential of precision medicine. As one example, in April 2023, FDA approved a combination of a molecularly targeted therapeutic, called enfortumab vedotin-ejfv (Padcev) and pembrolizumab to treat patients with bladder cancer. The approval was based on findings of a clinical study that showed that 73 percent of the treated patients responded to the drug combination and the response lasted, on average, for 22 months (433)Rosenberg JE, et al. (2023) J Clin Oncol, 41: 4505. [LINK NOT AVAILABLE], bringing hope to patients with bladder cancer who otherwise have limited treatment options available.
Research has shown that up to 60 percent of bladder cancers are characterized by elevated levels of a protein called nectin-4 (434)Challita-Eid PM, et al. (2016) Cancer Res, 76: 3003. [LINK NOT AVAILABLE]. Enfortumab-vedotin-ejfv is an antibody–drug conjugate that comprises a cytotoxic agent, monomethyl auristatin E, attached by a linker to a nectin-4–targeted antibody. When the antibody attaches to nectin-4 on the surface of bladder cancer cells, the antibody–drug conjugate is internalized by the cells, which leads to release of monomethyl auristatin E from the linker and antibody. Once free, monomethyl auristatin is toxic to cancer cells.
One of the new cancer types for which an ICI is now an approved treatment option is alveolar soft part sarcoma (ASPS). In December 2022, FDA approved the ICI atezolizumab (Tecentriq) for the treatment of adult and pediatric patients two years and older with ASPS that has spread to other parts of the body or cannot be removed by surgery.
Alveolar soft part sarcoma is an extremely rare cancer that mainly affects adolescents and young adults, such as Isabella (Bella) Snow Fraser and Alexis Browning. According to NCI, about 80 people are diagnosed with the disease in the United States each year. ASPS is a slow-growing cancer that forms in soft tissues such as muscle, fat, or nerves. Although the disease grows slowly, once metastatic, ASPS has poor outcomes. Chemotherapeutics have limited benefit and molecularly targeted therapeutics do not have lasting effectiveness against ASPS.
Atezolizumab is a PD-L1-targeting ICI that has been approved previously for the treatment of patients with several cancer types, including liver cancer, melanoma, and lung cancer (see Figure 19). The new FDA approval was based on data from a phase II clinical trial showing that about a quarter of the patients with ASPS responded to atezolizumab with some tumor shrinkage. Among patients who responded to the treatment, 67 percent had a response lasting at least six months, and 42 percent had a response lasting at least 12 months. The approval represents a significant advance for a rare disease as well as for pediatric patients with cancer, two research areas that require more intensive attention for continued progress.
In March 2023, retifanlimab-dlwr (Zynyz) became the second new ICI approved by FDA during the 12 months covered by the report. It was approved for the treatment of adult patients with metastatic or recurrent locally advanced Merkel cell carcinoma, a rare and aggressive form of skin cancer. Retifanlimab-dlwr targets the PD-1 brake on the surface of T cells, preventing T cells from attaching to the cells that can trigger the brake and deactivate them. The approval was based on the finding that treatment with retifanlimab-dlwr resulted in tumor shrinkage in more than half of the patients who received the treatment as part of a phase II clinical study. With this decision, retifanlimab-dlwr became the third ICI approved by the FDA for Merkel cell carcinoma (see Figure 19).
Immune checkpoint inhibitors have yielded extraordinary benefit for many patients, but they can have adverse effects (see Current Challenges in Cancer Immunotherapy) particularly the induction of autoimmune-like conditions. This occurs because ICIs not only release the brake on cancer fighting immune cells but also some that recognize and injure normal tissues. To predict which patients are likely to experience adverse events and design treatments to combat these events without compromising the anticancer efficacy of the ICI, researchers must better understand why and how the adverse effects arise. This is an area of extensive research investigation. Another important area of scientific inquiry is to identify behavioral and clinical factors, such as diet, physical activity, gut microbiome composition, and optimal combinations with other therapeutic modalities that can boost the efficacy of ICIs and increase the number of patients who respond favorably to these lifesaving treatments.
As documented in this report and in the past 12 editions of the AACR Cancer Progress Report, ICIs have transformed the clinical care of patients with a diverse array of cancer types including historically intractable diseases such as metastatic melanoma, lung cancer, and kidney cancer (436)Topalian SL, et al. (2019) JAMA Oncol, 5: 1411. [LINK NOT AVAILABLE]. While their use was initially limited to people with very advanced cancers that were no longer responding to standard treatments, checkpoint inhibitors are increasingly being approved as first-line—initial—treatments for patients. Recent data show that compared to the current standard treatments such as chemotherapy, ICI use in the first-line setting may improve survival for certain patients (437)Lamba N, et al. (2022) JAMA Netw Open, 5: e2225459. [LINK NOT AVAILABLE](438)Voruganti T, et al. (2023) JAMA Oncol, 9: 334. [LINK NOT AVAILABLE](439)LeBlanc ML, et al. (2023) J Clin Oncol, 41: LBA4. [LINK NOT AVAILABLE].
Researchers are also evaluating how to best integrate the use of ICIs along with standard treatments such as surgery, radiation therapy, and/or chemotherapy in patients with early-stage cancer (440)Schmid P, et al. (2020) N Engl J Med, 382: 810. [LINK NOT AVAILABLE](441)Altorki NK, et al. (2021) Lancet Oncol, 22: 824. [LINK NOT AVAILABLE]. One area of extensive research is the use of these therapeutics before initial surgery, known as neoadjuvant treatment, in people with locally advanced cancers that are largely restricted to their original location. The first neoadjuvant clinical trial with ICI was conducted in 2006, which provided the first safety data and evidence of clinical efficacy for ICI in the neoadjuvant setting (442)Carthon BC, et al. (2010) Clin Cancer Res, 16: 2861. [LINK NOT AVAILABLE](443)Liakou CI, et al. (2008) Proc Natl Acad Sci U S A, 105: 14987. [LINK NOT AVAILABLE]. These critical data set the stage for additional neoadjuvant clinical trials with ICI. Based on such studies FDA has approved the ICIs pembrolizumab and nivolumab as neoadjuvant treatments for patients with triple-negative breast cancer and lung cancer, respectively, that are diagnosed at an early stage.
The immense benefit of ICIs as neoadjuvant therapy was highlighted in two recent clinical trials conducted in patients with early-stage colorectal cancer that has specific genetic characteristics. Neoadjuvant treatment with either of the ICIs pembrolizumab or dostarlimab yielded remarkable responses, with some patients not even needing surgery and showing no evidence of cancer for years following the ICI therapy (444)Ludford K, et al. (2023) J Clin Oncol, 41: 2181. [LINK NOT AVAILABLE](445)Cercek A, et al. (2022) N Engl J Med, 386: 2363. [LINK NOT AVAILABLE]. A similar benefit of neoadjuvant ICI therapy has been noted in patients with melanoma, and those with cutaneous squamous cell carcinoma—the second most common type of skin cancer diagnosed in the United States— among other diseases (446)Patel S, et al. (2022) Annals of Oncology, 33: S1408. [LINK NOT AVAILABLE](447)Gross ND, et al. (2022) N Engl J Med, 387: 1557. [LINK NOT AVAILABLE].
Collectively, these results highlight a paradigm shift in the treatment of early-stage cancers, with researchers hypothesizing that ICI therapy alone may eliminate cancers for certain patients without the need for any further treatments. While these data bring new hope to the cancer community, before such approaches to ICI use can become standard of care, it is important that they are shown in rigorous, well-designed, large clinical trials to prevent/delay cancer recurrence and improve how long patients live. Ongoing research and future efforts will identify optimal biomarkers for determining patients who would most benefit from such novel ICI regimens.
Boosting the Cancer-killing Power of Immune Cells
Research has shown that immune cells, such as T cells, are naturally capable of destroying cancer cells. It has also shown that in patients with cancer, there are often insufficient numbers of cancer-killing T cells, and that the cancer-killing T cells that are present are unable to find or destroy the cancer cells for one of several reasons. This knowledge has led researchers to identify several ways to boost the ability of T cells to eliminate cancer cells.
Adoptive Cell Therapy
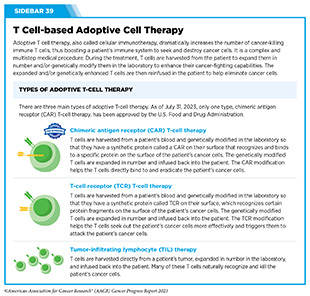
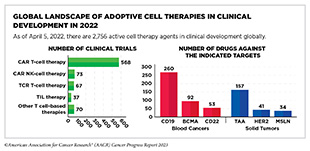
Adoptive cell therapy (ACT), also called cellular immunotherapy, is designed to dramatically increase the number of cancer-killing immune cells a patient has, thereby boosting the immune system’s ability to seek and destroy cancer cells (448)Rohaan MW, et al. (2019) Virchows Arch, 474: 449. [LINK NOT AVAILABLE]. While many of the adoptive cell therapies currently in late-stage clinical development and all that are approved by FDA utilize patient-derived T cells (see Sidebar 39), ongoing research is looking to harness the cancer killing power of other immune cell types including natural killer (NK) cells and macrophages, among others (see A New Wave of Adoptive Cell Therapeutics). Chimeric antigen receptor (CAR) T-cell therapy is one type of ACT that has generated enormous excitement in cancer medicine in recent years. This is because treatment with CAR T cells has demonstrated unprecedented efficacy in certain patients with very advanced leukemia or lymphoma.
Like ICIs, CAR T-cell therapy is the culmination of decades of basic, translational, and clinical research utilizing knowledge of the cellular and molecular components of the immune system, genetic engineering, and the biological underpinnings of blood cancers. The concept of a synthetic receptor was first reported in the late 1980s when researchers showed that the engineered proteins could be introduced into T cells and upon binding with appropriate partners could activate T cells, leading to killing of cancer cells (449)Mitra A, et al. (2023) Front Immunol, 14: 1188049. [LINK NOT AVAILABLE]. These discoveries, along with scientific breakthroughs in the 1990s on ways to expand T cells in large numbers in the laboratory and return them to the body where they could attack cancer cells, laid the foundation for the first clinical studies in the early 2010s that evaluated CAR T-cell therapy in patients with advanced leukemia (450)Brentjens RJ, et al. (2011) Blood, 118: 4817. [LINK NOT AVAILABLE](451)Porter DL, et al. (2011) N Engl J Med, 365: 725. [LINK NOT AVAILABLE](452)Grupp SA, et al. (2013) N Engl J Med, 368: 1509. [LINK NOT AVAILABLE].
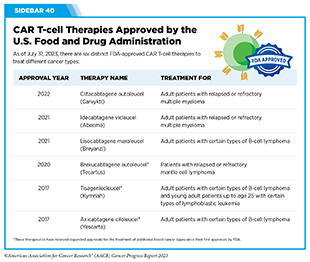
In 2017, tisagenlecleucel (Kymriah) became the first FDA approved CAR T-cell therapy when it was approved for the treatment of children and young adults with B-cell acute lymphoblastic leukemia that had not responded to standard treatments or had relapsed at least twice. The approval was based on results from a phase II clinical trial, showing that more than 80 percent of the children and young adults who were treated with tisagenlecleucel achieved a remission within three months of receiving the CAR T-cell therapy (453)Maude SL, et al. (2018) N Engl J Med, 378: 439. [LINK NOT AVAILABLE]. This revolutionary immunotherapeutic has allowed some children with leukemia, like Cayden Addison, to experience complete remission following treatment. In a recent study, long-term follow-up of patients treated with tisagenlecleucel showed that more than 60 percent were living three years or longer after their first infusion of CAR T cells, suggesting that CAR T cells can lead to durable cancer control (454)Laetsch TW, et al. (2023) J Clin Oncol, 41: 1664. [LINK NOT AVAILABLE].
Since 2017, five additional CAR T-cell therapies have been approved by FDA, all for the treatment of blood cancers, including NHL, leukemia, and most recently, multiple myeloma (see Sidebar 40). Collectively, these treatments have transformed the lives of adult and pediatric patients with blood cancers (455)Dana H, et al. (2021) Acta Pharm Sin B, 11: 1129. [LINK NOT AVAILABLE]. As one example, based on a recent analysis, axicabtagene ciloleucel (Yescarta), which was also approved by FDA in 2017, is the first treatment in nearly three decades to improve overall survival in relapsed or refractory large B-cell lymphoma (456)Westin JR, et al. (2023) N Engl J Med. [LINK NOT AVAILABLE].
Generating CAR T cells is a complex procedure that can only be performed at specially certified health care facilities by highly trained medical professionals. Additionally, like other cancer treatments, CAR T-cell therapies can cause side effects, some of which can be potentially life-threatening. Another issue that researchers are trying to address is the fact that CAR T-cell therapies have so far proven less successful against solid tumors. Developing simpler and safer ways to bring the promise of this class of immunotherapeutics to more patients with different cancer types is an area of active research (see On the Horizon for Immunotherapy).
Enhancing Immune Cell Function
Immune cells communicate with each other and with their surrounding cells by direct contact as well as through the release of a class of molecules called cytokines. Cytokines are also produced by nonimmune cells and play an essential role in rapidly activating the immune system in response to cellular stresses, such as infection, inflammation, and cancer (457)Liu C, et al. (2021) Adv Sci (Weinh), 8: e2004433. [LINK NOT AVAILABLE].
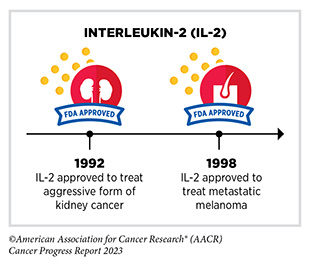
For many decades, researchers have been investigating the natural cancer killing ability of two cytokines, interferon-alpha (IFNα) and interleukin-2 (IL-2), to boost the cancer-killing function of the immune system (458)Conlon KC, et al. (2019) J Interferon Cytokine Res, 39: 6. [LINK NOT AVAILABLE]. Discoveries in the late 1960s established the role of interferons in suppressing tumor growth, which led to subsequent clinical trials confirming their anticancer effects (449)Mitra A, et al. (2023) Front Immunol, 14: 1188049. [LINK NOT AVAILABLE]. In 1986, IFNα became the first FDA-approved cancer immunotherapy when it was approved for the treatment of a rare blood cancer known as hairy-cell leukemia (459)Golomb HM, et al. (1986) J Clin Oncol, 4: 900. [LINK NOT AVAILABLE].
Although cytokines have shown some promise as immunotherapeutics, their success has been limited. One limitation is that cytokines do not persist very long in the body, so ongoing research is developing more stable versions of cytokines (461)Xue D, et al. (2021) Antib Ther, 4: 123. [LINK NOT AVAILABLE]. Another challenge is the significant adverse effects when cytokines are administered as a systemic treatment. Researchers are exploring ways to enhance the efficacy of interferons while minimizing their side effects, for example, by delivering them in or near tumors (461)Xue D, et al. (2021) Antib Ther, 4: 123. [LINK NOT AVAILABLE].
The FDA approval of nadofaragene firadenovec-vncg (Adstiladrin) in December 2022, was a major advance in the field of interferon-based cancer immunotherapy. The FDA approved nadofaragene firadenovec-vncg for the treatment of adult patients with high-risk, non–muscle-invasive bladder cancer (NMIBC) that did not respond to Bacillus Calmette-Guérin (BCG) treatment.
More than 82,000 new cases of bladder cancer will be diagnosed in the United States in 2023 (28)American Cancer Society. Cancer Facts and Figures. Accessed: July 5, 2023. . Non–muscle-invasive bladder cancer—a type of cancer that has grown through the lining of the bladder but hasn’t yet invaded the muscle layer—makes up around 75 percent of all new cases of bladder cancer (462)Mond HG, et al. (1981) Pacing Clin Electrophysiol, 4: 304. [LINK NOT AVAILABLE]. Patients with high-risk NMIBC are usually treated with BCG—an immunotherapeutic that was originally developed as a vaccine against tuberculosis (TB)—which is instilled directly into the bladder. While 80 percent of patients initially respond to BCG, within a year over half of patients with an initial response experience recurrence and progression of cancer, and many develop disease that becomes BCG-unresponsive (463)Bree KK, et al. (2021) Hematol Oncol Clin North Am, 35: 513. [LINK NOT AVAILABLE](464)Boorjian SA, et al. (2021) Lancet Oncol, 22: 107. [LINK NOT AVAILABLE].
Patients who do not respond to BCG have very few treatment options other than surgical removal of the bladder. Although potentially curative, such surgical therapy is associated with high rates of complication. Additionally, many patients with underlying health conditions are unwilling or unable to undergo the surgery (464)Boorjian SA, et al. (2021) Lancet Oncol, 22: 107. [LINK NOT AVAILABLE]. Therefore, alternative treatments for patients with bladder cancer, such as Lesa Kirkman, are an urgent medical need, and are addressed by the nadofaragene firadenovec-vncg approval.
Nadofaragene firadenovec-vncg is a novel therapeutic. It is the first gene therapy approved for bladder cancer. When instilled into the bladder through a urinary catheter, the therapeutic infiltrates the bladder cells and delivers a gene encoding interferon alpha 2b (IFNα2b) into the DNA of the cells it infiltrates. Once the gene is incorporated into the bladder cells, they produce the IFNα2b protein, which blocks bladder cancer growth through the activation of immune cells, among other mechanisms (466)Lee A (2023) Drugs, 83: 353. [LINK NOT AVAILABLE]. The approval was based on a Phase II, single arm study with registration intent. Findings of the study showed that among 51 percent of patients treated with nadofaragene firadenovec-vncg no cancer cells could be detected in their urine and in the urinary bladder tissue (464)Boorjian SA, et al. (2021) Lancet Oncol, 22: 107. [LINK NOT AVAILABLE].
Researchers are also evaluating IFNα2b treatment as a potential therapy for other cancer types. As one example, recent data show that people with low-grade lymphomatoid granulomatosis, a rare condition that can progress into an aggressive B-cell lymphoma, can live for decades after diagnosis when treated with IFNα2b (467)Melani C, et al. (2023) Lancet Haematol, 10: e346. [LINK NOT AVAILABLE]. This is a remarkable advance compared to findings from past studies showing median survival of less than two years for people with lymphomatoid granulomatosis.
Directing the Immune System to Cancer Cells
An immune cell must find a cancer cell before it can attack and eliminate it. Many therapeutic antibodies approved by FDA for the treatment of various types of cancer work, at least in part, by helping immune cells find cancer cells. Because of the effectiveness and promise of antibody-based immunotherapeutics, many researchers have been working to develop new as well as improved versions of this important class of anticancer therapeutics.
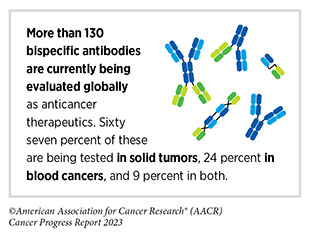
One class of recently developed therapeutic antibodies is known as T-cell engaging bispecific antibodies. Using two or more arms that are engineered into these antibody particles, these immunotherapeutics bind to immune cells and cancer cells simultaneously. By acting as a connector, T-cell engaging bispecific antibodies bring cancer cells into close proximity with T cells, which are then activated and eliminate the cancer cells.
The first of these agents, blinatumomab (Blincyto), was approved by FDA in December 2014 for treating certain patients with a type of acute lymphoblastic leukemia (ALL) called B-cell ALL (468)Baselga J, et al. (2015) Clin Cancer Res, 21: S1. [LINK NOT AVAILABLE]. Unprecedented advances in genetic engineering, molecular biology, and immunology over the past decade have led to a rapid proliferation in this innovative new area of cancer medicine. Between August 1, 2022, and July 31, 2023, FDA approved four new T-cell engaging bispecific antibodies for the treatment of a number of hematologic cancers.
In October 2022, FDA approved teclistamab-cqyv (Tecvayli) for adult patients with multiple myeloma that has relapsed after, or never responded to, at least four prior lines of therapy. Teclistamab-cqyv attaches to a molecule called CD3 on T cells with one arm and to B-cell maturation antigen (BCMA), a molecule that is abundant on the surface of most multiple myeloma cells, with the second arm. By attaching to these molecules on different cells, teclistamab-cqyv brings the two cell types together, directing the T cells to home in on the myeloma cells. As a result, T cells are activated and they destroy the adjacent myeloma cells.
The FDA approval of teclistamab-cqyv was based on data from a phase I/II clinical trial that evaluated the immunotherapeutic in 110 patients who had stopped responding to at least three classes of drugs prior to receiving teclistamab-cqyv (470)Moreau P, et al. (2022) N Engl J Med, 387: 495. [LINK NOT AVAILABLE]. More than 60 percent of participants responded to therapy with teclistamab-cqyv (470)Moreau P, et al. (2022) N Engl J Med, 387: 495. [LINK NOT AVAILABLE]. Historically, patients with multiple myeloma that has progressed following multiple classes of treatment have been extremely challenging to treat. In fact, with immunotherapeutics such as daratumumab, which is approved for these patients, response is seen only in 30 percent of patients or fewer (471)Leslie M (2023) Cancer Discov, 13: OF1. [LINK NOT AVAILABLE]. Therefore, the approval of teclistamab-cqyv brings hope to patients like Cindy Brown by providing them with a new and effective treatment option.
It is important to note that some of these immunotherapeutics can have life-threatening side effects if not managed immediately and appropriately by trained medical professionals. For example, FDA approval of teclistamab-cqyv comes with a warning of life-threatening adverse events, such as cytokine release syndrome and neurologic toxicity, which are usually manageable. Because of these risks, teclistamab-cqyv is available only through a restricted program, called the Tecvayli Risk Evaluation and Mitigation Strategy (REMS).
The three other T-cell engaging bispecific antibodies approved by FDA during the 12 months covered in this report—mosunetuzumab-axgb (Lunsumio), epcoritamab-bysp (Epkinly), and glofitamab-gxbm (Columvi)—all work by attaching to CD3 on T cells and to CD20, a protein that is found in abundance on the surface of cancerous B cells. By bringing the two cell types together, these therapeutics help activate the T cells to destroy cancerous B cells.
Mosunetuzumab-axgb was approved by FDA in December 2022 for the treatment of certain patients with relapsed or refractory follicular lymphoma, the second most common form of NHL diagnosed in the United States. Follicular lymphoma is a slow-growing cancer that arises in immune cells called B cells (see Sidebar 38) but can eventually progress to become a fatal disease. Most B cells, including follicular lymphoma B cells, have a protein called CD20 on their surface, making CD20-targeted therapeutic antibodies an attractive treatment option for this cancer.
CD20-targeting therapeutic antibodies have been approved previously by FDA for the treatment of follicular lymphoma and have a very high response rate. Rituximab (approved in 2006) and obinutuzumab (approved in 2016) are two examples. However, it should be noted that these antibodies work differently from mosunetuzumab-axgb. Mosunetuzumab-axgb is the first T-cell engaging bispecific antibody approved for follicular lymphoma.
The FDA approval of mosunetuzumab-axgb was based on results from a phase I/II clinical trial in which the therapeutic was given to 90 patients with follicular lymphoma that had relapsed or stopped responding to other treatments. All patients had received at least two prior treatments, including a CD20-targeting therapeutic antibody. The results from the trial showed that 80 percent of patients whose disease was not responding to prior treatments had partial or complete tumor shrinkage, with 60 percent exhibiting complete shrinkage following treatment with mosunetuzumab-axgb (472)Budde LE, et al. (2022) Lancet Oncol, 23: 1055. [LINK NOT AVAILABLE]. The complete response rate was significantly higher than that observed with current standard treatments. The approval of mosunetuzumab-axgb comes with a warning for cytokine release syndrome, which may occur as a result of the hyperactivation of T cells that are targeting the lymphoma.
Both epcoritamab-bysp and glofitamab-gxbm were approved for the treatment of patients with certain types of NHL. Diffuse large B-cell lymphoma (DLBCL) and high-grade B-cell lymphoma are types of NHL that arise from B cells. DLBCL is the most common form of the disease, accounting for 30 to 40 percent of all cases (473)Sethi A, et al. (2019) J Oral Maxillofac Pathol, 23: 284. [LINK NOT AVAILABLE]. DLBCL often develops from evolution of other types of lymphoma, including follicular lymphoma, that have been undergoing therapy. Different subtypes of DLBCL exist. When a patient does not fit into any of those subtypes, it is classified as DLBCL not otherwise specified.
Epcoritamab-bysp was approved by FDA in May 2023 for patients with either DLBCL not otherwise specified or high-grade B-cell lymphoma whose disease did not respond to or relapsed following two or more lines of systemic therapy. The approval was based on results from a phase I/II clinical trial which showed that 61 percent of patients responded to the therapeutic and 38 percent of patients achieved complete responses with no signs or symptoms of cancer following the treatment. The approval of epcoritamab-bysp includes a warning that the treatment can cause serious or life-threatening immune-related adverse reactions and should only be administered by qualified health care professionals with appropriate medical support.
Glofitamab-gxbm was approved in June 2023 for patients with DLBCL, not otherwise specified or large B-cell lymphoma arising from follicular lymphoma, who relapsed after or did not respond to two or more lines of systemic therapy. The approval was based on data from a phase I/II study in which more than half of all patients responded to the treatment, with approximately 40 percent achieving complete responses, meaning researchers could not detect signs or symptoms of cancer (474)Dickinson MJ, et al. (2022) N Engl J Med, 387: 2220. [LINK NOT AVAILABLE].
Glofitamab-gxbm has a few unique characteristics that set it apart from the other T-cell engaging bispecific antibodies discussed in this section. First, glofitamab-gxbm is approved to be administered for a fixed duration of about 8.5 months, whereas most other agents are administered until the disease progresses or the therapeutic cannot be tolerated any longer. The fixed-duration approach is used because data indicate that administration until disease progression is not required to achieve a durable remission with glofitamab. The fixed-duration approach is attractive to patients because it provides them with a shorter and potentially less toxic treatment option. Second, glofitamab-gxbm has two arms to latch onto CD20 unlike epcoritamab-bysp and mosunetuzumab-axgb, which only have one, and is therefore expected to be more potent. The approval of glofitamab-gxbm includes a warning that the treatment can cause serious or life-threatening immune-related adverse reactions and should only be administered by qualified health care professionals with appropriate medical support.
While ongoing research is needed to identify ways to minimize the adverse effects, T-cell engaging bispecific antibodies are now offering a wide selection of new therapeutics and providing hope to many patients with advanced cancer who are often out of options and urgently need effective treatments for their rapidly progressing disease.
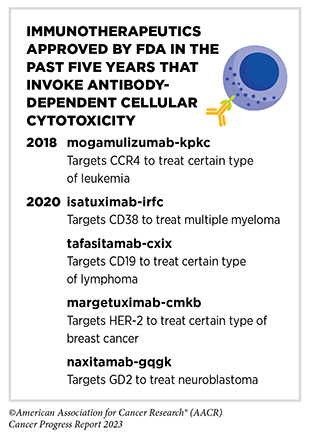
Another group of therapeutic antibodies that mark cancer cells for elimination by the immune system uses a natural process called antibody-dependent cellular cytotoxicity. When the immune system detects a pathogen or damaged cells, B cells produce antibodies that flag unwanted cells or organisms, which are then recognized and killed by immune cells such as NK cells (see Sidebar 38) (475)Zahavi D, et al. (2018) Antib Ther, 1: 7. [LINK NOT AVAILABLE]. Researchers are using this knowledge to develop antibodies that bind to specific targets on cancer cells and invoke antibody-dependent cellular cytotoxicity to kill them.
Many therapeutic antibodies that work this way have been approved by FDA and are benefiting numerous patients with different cancer types including solid tumors, hematologic cancers, and pediatric cancers. As one example, dinutuximab (Unituxin), which was approved in 2015 for treating children with high-risk neuroblastoma, works by attaching to a protein, GD2, on neuroblastoma cells and flagging them for destruction by immune cells. Decades of basic, translational, and clinical research, starting from the recognition of GD2 as a tumor protein in 1984, led to the development of dinutuximab. Recent data demonstrate that the therapeutic antibody is extending lives for many children with high-risk neuroblastoma (476)Desai AV, et al. (2022) J Clin Oncol, 40: 4107. [LINK NOT AVAILABLE]. Since the approval of dinutuximab in 2015, the FDA has approved a second therapeutic, naxitamab-gqgk (Danyelza), that works similarly to dinutuximab, for the treatment of patients with neuroblastoma.
Current Challenges in Cancer Immunotherapy
As described throughout this section, over the past decade, immunotherapeutics have revolutionized the landscape of cancer treatment. However, immunotherapeutics have been successful in treating only a fraction of patients with cancer with several challenges remaining. These include serious and even life-threatening adverse effects; patients who respond initially but develop resistance over time; and the current gaps in our understanding of how to integrate immunotherapies with standard cancer therapies. In this section we outline some of the current limitations and known challenges of cancer immunotherapy.
Knowledge Gaps in Predicting Response to Immunotherapy
Immunotherapeutics have shown extremely durable responses in certain patients with cancer. To determine if a patient may respond to these therapeutics, clinicians use biomarkers, molecules that can identify certain characteristics of a cell or a tissue. The most common biomarkers currently used to select patients for ICI treatment are the presence of certain surface proteins on cancer cells and the presence of specific molecular characteristics.
The presence of a biomarker, for example surface levels of PD-L1 protein, informs the clinician whether a patient might respond to a PD-1/PD-L1 targeted immunotherapy, such as pembrolizumab. However, one concern is that levels of these biomarkers are not always consistent with one study finding that levels of PD-L1 change over time and are not the most reliable biomarker for anti-PD-1 therapies (477)Grossman JE, et al. (2021) Oncogene, 40: 1393. [LINK NOT AVAILABLE].
Another major challenge in cancer immunology biomarker research is the lack of diversity in genetic databases and underrepresentation of individuals of non-European genetic ancestry (478)Aldrighetti CM, et al. (2021) JAMA Netw Open, 4: e2133205. [LINK NOT AVAILABLE]. As a result, gaps remain in our understanding of the ancestry-related differences of tumors and the immune system, which are key contributing factors in determining efficacy of cancer immunotherapies. As one example, one study found that Black individuals can be misclassified as having a cancer with a high tumor mutational burden (TMB-h), a biomarker used in some cases to select patients for treatment with certain immunotherapeutics, including pembrolizumab. Further, the study found that patients of African ancestry classified as h-TMB did not respond to pembrolizumab because of this misclassification (479)Nassar AH, et al. (2022) Cancer Cell, 40: 1161. [LINK NOT AVAILABLE]. These findings illustrate the importance of increasing diversity of genetic databases to include more individuals from non-European ancestries.
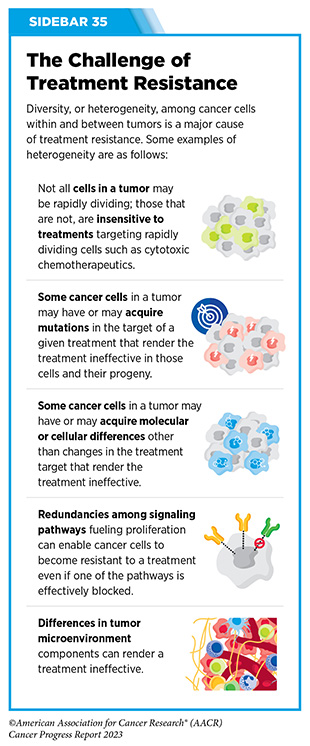
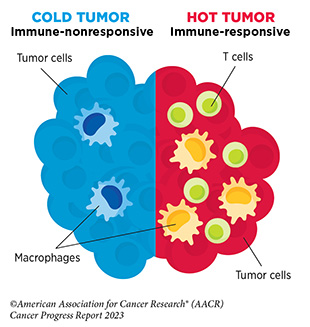
Like other anticancer therapeutics, one concern after ICI treatment is the possibility of cancer recurrence. Recurrence or relapse of metastatic disease after initial response may occur because of acquired resistance, wherein a cancer no longer responds to the therapeutic (see Sidebar 35). The underlying mechanism of acquired resistance is multifactorial and is influenced by both extrinsic and intrinsic factors (480)Jenkins RW, et al. (2018) Br J Cancer, 118: 9. [LINK NOT AVAILABLE]. Extrinsic factors include the tumor microenvironment (see Tumor Microenvironment), and the presence of certain inhibitory immune cells that can change or accumulate over time. Intrinsic factors that lead to acquired resistance of a tumor include the downregulation of certain proteins on the surface of cancer cells that are required for the therapeutic to work. Identifying biomarkers that predict cancer recurrence is an area of active research. Utilization of combination therapy and strategies that target the tumor microenvironment are among the ways to overcome resistance and is another area of extensive investigation (see A New Age of Therapeutic Combinations).
Finally, when a patient should cease receiving treatment with an ICI has not been well defined. This is because traditional endpoints, such as overall survival, progression free survival, and overall response rate, are not well-matched for assessment of ICIs (481)Gauci ML, et al. (2019) Clin Cancer Res, 25: 946. [LINK NOT AVAILABLE](482)Hegde PS, et al. (2020) Immunity, 52: 17. [LINK NOT AVAILABLE]. For instance, when measuring the overall response rate of a tumor to therapy, clinicians often examine tumor size before and after a treatment. Tumor shrinkage after therapy corresponds to a higher overall response rate. However, with ICI treatment, tumors often continue to grow before they shrink (e.g., pseudoprogression), which may initially be perceived as a lack of response (483)Hodi FS, et al. (2016) J Clin Oncol, 34: 1510. [LINK NOT AVAILABLE]. To overcome these limitations, criteria to define endpoints for ICIs have been developed (484)Claret L, et al. (2018) Clin Cancer Res, 24: 3292. [LINK NOT AVAILABLE]; however, validation of these criteria will require large amounts of patient-derived data over a long period of time. Continued optimization and research for ICI endpoints must be developed to understand how long patients need to continue treatment.
Adverse Effects of Immunotherapy
Immunotherapeutics have led to more cancer survivors living through and beyond their cancer, with some patients remaining cancer free for 10 or more years. As the use of immunotherapy becomes more widespread, understanding the immediate and long-term adverse health impact of these therapeutics is crucial to improve health outcomes.
Cytokine release syndrome (CRS) is the most common immediate side effect following treatment with CAR T-cell therapy with a reported incidence of 37-93 percent (453)Maude SL, et al. (2018) N Engl J Med, 378: 439. [LINK NOT AVAILABLE](485)Messmer AS, et al. (2021) Wien Klin Wochenschr, 133: 1318. [LINK NOT AVAILABLE](486)Neelapu SS, et al. (2017) N Engl J Med, 377: 2531. [LINK NOT AVAILABLE]. CRS can develop within hours or days following infusion and is characterized by an excessive immune reaction that leads to widespread organ dysfunction that can become life-threatening (485)Messmer AS, et al. (2021) Wien Klin Wochenschr, 133: 1318. [LINK NOT AVAILABLE]. Following CRS, up to 40 percent of CAR T-cell recipients may develop another type of side effect called immune effector cell-associated neurotoxicity syndrome (ICANS), which can lead to neurological symptoms that affect speech, orientation, handwriting, and concentration (487)Gust J, et al. (2017) Cancer Discov, 7: 1404. [LINK NOT AVAILABLE].
While alarming, these side effects are often short-lived and readily managed with both corticosteroids and the IL-6R antagonist tocilizumab, approved by FDA to treat CRS in 2017 (488)Maude SL, et al. (2014) Cancer J, 20: 119. [LINK NOT AVAILABLE](489)Sengupta R, et al. (2018) Clin Cancer Res, 24: 4351. [LINK NOT AVAILABLE]. Further evidence demonstrates that once a patient overcomes these symptoms, they do not recur (490)Shimabukuro-Vornhagen A, et al. (2018) J Immunother Cancer, 6: 56. [LINK NOT AVAILABLE].
A long-term side effect that has been observed in patients who receive CAR T-cell therapy targeting B-cell malignancies is depletion of normal B cells, called B cell aplasia. This condition arises when CAR T-cell therapy targets CD19, which is expressed not only on cancer cells but also on nonmalignant B cells, meaning that these cells can also be eliminated. B cell aplasia has been reported in 25-38 percent of patients for up to several years after conclusion of the treatment (491)Cappell KM, et al. (2023) Nat Rev Clin Oncol, 20: 359. [LINK NOT AVAILABLE](492)Cappell KM, et al. (2020) J Clin Oncol, 38: 3805. [LINK NOT AVAILABLE].
Other long-term side effects of CAR T-cell therapy include cytopenias, which are conditions in which there are lower than normal numbers of blood cells. Cytopenias include anemia (low red blood cells), thrombocytopenia (low platelets), and neutropenia (low neutrophils), and can occur in approximately 15 percent of patients with B-cell lymphoma more than three months after CAR T-cell infusion (493)Brudno JN, et al. (2019) Blood Rev, 34: 45. [LINK NOT AVAILABLE](494)Brudno JN, et al. (2016) Blood, 127: 3321. [LINK NOT AVAILABLE](495)Locke FL, et al. (2019) Lancet Oncol, 20: 31. [LINK NOT AVAILABLE](496)Logue JM, et al. (2021) Haematologica, 106: 978. [LINK NOT AVAILABLE]. Cytopenias persisted in long-term follow-up of 15–22 months in about 16 percent of patients (497)Cordeiro A, et al. (2020) Biol Blood Marrow Transplant, 26: 26. [LINK NOT AVAILABLE].
Other reported side effects, including increased risk of infection and malignant transformation of transduced cells are not well understood. Further complicating these findings is the difficulty of attributing these side effects to the treatment or the cancer itself.
The development of immune-related adverse events (irAEs), which are side effects from treatment with ICIs, can occur from the beginning of treatment and last well beyond its completion. The likelihood of someone treated with ICIs developing at least one irAE varies between 13.7 percent and 54 percent depending on the type of ICI (498)Aggarwal C, et al. (2016) Annals of Oncology, 27. [LINK NOT AVAILABLE].
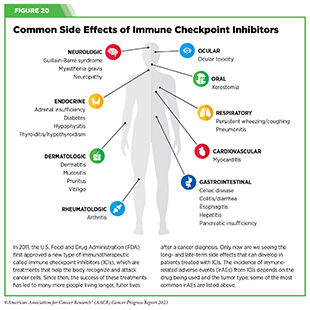
Although they are often mild, ICI-induced irAEs can develop into more serious conditions that could become irreversible or even fatal (499)Postow MA, et al. (2018) N Engl J Med, 378: 158. [LINK NOT AVAILABLE]. Many of these side effects are similar to autoimmune reactions, such as colitis, rash, or arthritis. These side effects have been documented in many organs and tissues (see Figure 20).
Identification of new biomarkers is vital to better predict if a patient will develop irAEs, as well as to reduce the possibility of these side effects. One promising area is examining whether the levels of certain immune cells in the body are associated with the development of severe irAEs. One study showed that levels of certain types of CD8+ T cells could predict if a patient would develop arthritis following the administration of ICIs (see Sidebar 38) (503)Mor A, et al. (2021) Front Cell Dev Biol, 9: 790386. [LINK NOT AVAILABLE]. The presence of certain immune cells has also been associated with the development of side effects such as colitis, pneumonitis, and thyroiditis (503)Mor A, et al. (2021) Front Cell Dev Biol, 9: 790386. [LINK NOT AVAILABLE](504)Cardena-Gutierrez A, et al. (2022) Front Med (Lausanne), 9: 908752. [LINK NOT AVAILABLE](505)Chaput N, et al. (2017) Ann Oncol, 28: 1368. [LINK NOT AVAILABLE]. Biomarkers in the blood could also be used to predict severe irAE, including cytokines such as IL-17, IL-6, and IL-8, all of which are elevated in patients who develop colitis following treatment with ICIs (505)Chaput N, et al. (2017) Ann Oncol, 28: 1368. [LINK NOT AVAILABLE](506)Manson G, et al. (2016) Ann Oncol, 27: 1199. [LINK NOT AVAILABLE].
As patients treated with ICIs are living longer (507)Martins F, et al. (2019) Nat Rev Clin Oncol, 16: 563. [LINK NOT AVAILABLE], it is important to note that although oncologists are generally aware of the potential for irAEs, this awareness is not as prevalent among primary care physicians who are a patient’s first point of contact when seeking care. One study found that while 93 percent of physicians recognized the gastrointestinal tract was at risk for developing irAEs, only 57 percent recognized that the cardiovascular system and 67 percent recognized that the renal system was at risk following administration of ICIs (508)Khalid AB, et al. (2022) J Natl Compr Canc Netw, 20: 1316. [LINK NOT AVAILABLE].
With the increased use of immunotherapeutics resulting in improved survival, a better understanding of their long-term and late effects is urgently needed to improve quality of life for patients who receive immunotherapy.
Disparities in Access to Immunotherapy
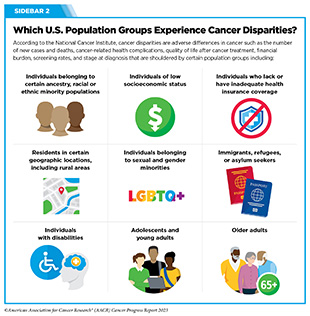
Emerging evidence shows that there are disparities in the access to immunotherapeutics, such as ICIs, particularly among racial and ethnic minority groups (see Sidebar 2). One study of over 17,000 patients found that counties that were urban, had a higher proportion of Hispanic and Latino populations and higher poverty levels, had slower initiation of ICI therapy after diagnosis (510)Li M, et al. (2023) J Natl Cancer Inst, 115: 295. [LINK NOT AVAILABLE]. The slow uptake of ICI occurred despite a higher density of cancer physicians, which indicates that the disparity was not exclusively due to lack of availability of appropriate care facilities.
Treatment of HCC, with immunotherapeutics was lower among both Hispanic and Black patients compared to White patients despite HCC having a higher incidence among Black and Hispanic populations (511)Muhimpundu S, et al. (2021) Cancers (Basel), 13. [LINK NOT AVAILABLE](512)Ahn JC, et al. (2022) Hepatology. [LINK NOT AVAILABLE]. This is especially alarming because access to ICI therapy has been shown to improve overall survival compared to other types of treatment, such as chemotherapy (512)Ahn JC, et al. (2022) Hepatology. [LINK NOT AVAILABLE].
There are also socioeconomic disparities in the access to immunotherapies. In a recent study examining immunotherapy use among patients with advanced-stage NSCLC, researchers found that having a low income and a lower level of educational attainment were associated with lower likelihood of receiving immunotherapy, and this persisted regardless of race or ethnicity (353)Gupta A, et al. (2023) Sci Rep, 13: 8190. [LINK NOT AVAILABLE].
There are also disparities in access to adoptive cell therapies. One study that evaluated the geographic distribution of CAR T-cell clinical trials for multiple myeloma found that 34 percent of states analyzed had no CAR T-cell or bispecific antibody clinical trial openings, with limited sites in states that have the highest percentages of Black residents (513)Alqazaqi R, et al. (2022) JAMA Netw Open, 5: e2228877. [LINK NOT AVAILABLE]. These data may explain why only two to five percent of participants in the pivotal clinical trials that led to the FDA approval of the CAR T-cell therapy tisagenlecleucel for ALL were Black (514)Al Hadidi S, et al. (2022) JAMA Netw Open, 5: e228161. [LINK NOT AVAILABLE].
Inequities in the current utilization of these highly effective therapies mandate further research to identify current barriers to the use of immunotherapy among medically underserved patients with cancer and to develop ways to address those barriers at the earliest possible time.
On the Horizon for Immunotherapy
As highlighted throughout this section, cancer immunotherapy has revolutionized cancer treatment and brought hope to countless patients with cancer who otherwise have no or limited treatment options. Thanks to research, progress in the field of immunotherapy is continuing at an unprecedented pace. Researchers are exploring several exciting new avenues to increase the number of cancer types that are treatable with immunotherapy, some of which are outlined in this section.
A New Era of mRNA-based Cancer Vaccines
Cancer vaccines work by boosting the magnitude of a patient’s T cells with natural cancer-killing capacity. They do this by providing large amounts of the small pieces of cancer-specific proteins that T cells need to recognize on the surface of tumor cells in order to eliminate the cancer cells (515)Xie N, et al. (2023) Signal Transduct Target Ther, 8: 9. [LINK NOT AVAILABLE]. Although the concept of cancer vaccines is not new, developing effective cancer vaccines has been challenging, as evidenced by the fact that, as of July 31, 2023, there is only one FDA approved therapeutic cancer vaccine (516)Grimmett E, et al. (2022) Discov Oncol, 13: 31. [LINK NOT AVAILABLE].
The success of mRNA-based vaccines against SARS-CoV-2— the virus that caused the COVID-19 pandemic—has renewed interest in using mRNA-based vaccines to treat cancer (517)Saxena M, et al. (2021) Nat Rev Cancer, 21: 360. [LINK NOT AVAILABLE]. Furthermore, exciting new technological advances in sequencing DNA, RNA, and proteins are helping to catalogue abnormal proteins present in tumor cells that represent potential targets. Researchers are leveraging this knowledge to develop mRNA-based cancer vaccines that are tumor and patient specific.
The enormous potential of cancer vaccines is underscored by the findings of a recent study in pancreatic cancer, a particularly aggressive disease (518)Rojas LA, et al. (2023) Nature, 618: 144. [LINK NOT AVAILABLE]. Researchers developed an mRNA-based cancer vaccine that was tailored toward the abnormalities in the tumors in each of the 16 patients with pancreatic cancer who participated in the study. The vaccine stimulated an immune response against the cancer in half of the patients, and those who responded to the treatment did not have signs of cancer at the 18-months follow-up. These findings are extremely promising because pancreatic cancer has very low survival rate and very few treatment options (518)Rojas LA, et al. (2023) Nature, 618: 144. [LINK NOT AVAILABLE]. Another recent study evaluated the effectiveness of an mRNA-based vaccine for the treatment of metastatic melanoma. The results showed that the combination of the vaccine and the ICI pembrolizumab reduced the likelihood of cancer recurrence or death by nearly 44 percent compared to pembrolizumab treatment alone, which is a current standard of care for these patients (519)Mørk SK, et al. (2023) J Clin Oncol, 41: 9551. [LINK NOT AVAILABLE].
Cancer cells change constantly as cancer progresses (see Understanding the Path to Cancer Development). As a result, the types of alterations in tumor proteins, as well as protein fragments present on the surface of tumor cells, also change. Advantages of mRNA-based cancer vaccines are that these therapeutics can induce responses to multiple targets, thus addressing heterogeneity, can be tailored against a patient’s changing tumor, and can be produced rapidly (520)Huff AL, et al. (2022) J Clin Invest, 132. [LINK NOT AVAILABLE]. This latter capability allows researchers to periodically monitor which protein pieces are present on the surface of tumor cells of a patient, and to develop new versions of the vaccines that are more effective.
Therapeutic mRNA-based cancer vaccines have not yet been FDA approved for cancer treatment. However, a recent review of the early clinical studies evaluating the potential of mRNA-based vaccines in treating various types of cancer shows that findings from many of these ongoing studies are encouraging (521)Lorentzen CL, et al. (2022) Lancet Oncol, 23: e450. [LINK NOT AVAILABLE], and point to exciting new developments in this area of cancer medicine.
A New Generation of Immune Checkpoint Inhibitors
As discussed earlier, ICIs are one of the most widely effective cancer immunotherapies. As of July 31, 2023, FDA has approved 11 ICIs for the treatment of 20 cancer types (see Figure 20). Although ICI treatment can lead to better long-term outcomes compared to other anticancer agents for certain patients, most patients do not respond to ICIs, and many who do respond at first eventually develop resistance to the treatment (522)Karasarides M, et al. (2022) Cancer Immunol Res, 10: 372. [LINK NOT AVAILABLE].
Researchers are continually working to develop a much deeper understanding of additional immune checkpoint proteins and how they may be targeted alone or in combinations for cancer treatment. Progress in this area is reflected by the 2022 FDA approval of an ICI targeting a new immune checkpoint protein, called LAG-3 (1)American Association for Cancer Research. AACR Cancer Progress Report 2022. Accessed: July 5, 2023. . Researchers are investigating the therapeutic potential of targeting other immune checkpoint proteins, such as B and T lymphocyte attenuator (BTLA) (524)Pardoll DM (2012) Nature Reviews Cancer, 12. [LINK NOT AVAILABLE], T-cell immunoglobulin and mucin domain-containing protein 3 (TIM3) (525)Acharya N, et al. (2020) J Immunother Cancer, 8. [LINK NOT AVAILABLE], V-domain Ig suppressor of T-cell activation (VISTA) (526)Tagliamento M, et al. (2021) Immunotargets Ther, 10: 185. [LINK NOT AVAILABLE], and T-cell immunoreceptor with Ig and ITIM domains (TIGIT) (527)Chauvin JM, et al. (2020) J Immunother Cancer, 8. [LINK NOT AVAILABLE], to unleash the immune system against tumors. Of these ICIs, researchers have identified TIGIT as one of the most promising new targets for cancer immunotherapy.
TIGIT is present on T cells and NK cells (see Sidebar 38), and functions as a brake on the immune system. As one example, initial findings from an ongoing phase II clinical trial evaluating the potential of one such TIGIT inhibitor, tiragolumab, showed promising results in treating patients with lung cancer (528)Cho BC, et al. (2022) Lancet Oncol, 23: 781. [LINK NOT AVAILABLE]. In another phase II clinical trial, treatment of patients with stage IV lung cancer with a different TIGIT inhibitor, domvanalimab, showed improvement in progression-free survival (see Sidebar 32) (529)Johnson ML, et al. (2022) J Clin Oncol, 40: 397600. [LINK NOT AVAILABLE]. It is important to note that these are still preliminary data and larger, randomized phase III studies are needed to establish whether TIGIT inhibitors will improve health outcomes for patients with cancer. There are currently 85 ongoing clinical studies evaluating more than 15 inhibitors of TIGIT at various phases of clinical development (530)Chiang EY, et al. (2022) J Immunother Cancer, 10. [LINK NOT AVAILABLE]. Findings from these and future studies will provide further insight into the effectiveness of TIGIT inhibitors in cancer immunotherapy.
Researchers are also evaluating ICIs that can release the brakes on other types of immune cells beyond T cells and unleash them against cancer. As one example, research has shown that macrophages (see Sidebar 38) have a protein, called signal regulatory protein α (SIRPα), on their surface. SIRPα functions as a brake on macrophages and stops them from killing microorganisms, removing dead cells, or activating other immune cells. Interestingly, many tumor cells have high amounts of a protein, called CD47, on their surface, which binds to SIRPα, and sends the “do not eat me” signal to macrophages (531)Chao MP, et al. (2012) Curr Opin Immunol, 24: 225. [LINK NOT AVAILABLE]. Ongoing research is focused on developing drugs that can release the interaction between the two proteins and activate macrophages against different types of cancer (532)Chao MP, et al. (2019) Front Oncol, 9: 1380. [LINK NOT AVAILABLE](533)Jiang Z, et al. (2021) J Hematol Oncol, 14: 180. [LINK NOT AVAILABLE].
A New Wave of Adoptive Cell Therapeutics
While all of the ACTs currently approved by FDA are based on CAR T cells (see Sidebar 40), extensive ongoing research is exploring the utility of other ways to modify T cells (534)Shafer P, et al. (2022) Front Immunol, 13: 835762. [LINK NOT AVAILABLE], as well as of other types of immune cells as anticancer agents.
One group of immune cells with therapeutic potential against cancer are called natural killer (NK) cells. NK cells act rapidly and directly to kill infected, damaged, or cancer cells by releasing toxic compounds. This efficiency and speed of killing unwanted cells make NK cells an ideal candidate for developing effective immunotherapeutics.
Compared to the currently approved CAR T-cell therapies, immunotherapeutics based on NK cells have several advantages including relatively better availability (NK cell-based treatments do not necessarily rely on cells isolated from patients; cells could potentially be isolated from healthy individuals), comparatively shorter time to be available for therapy (NK cells can be prepared and stored for later use), and potentially fewer side effects (536)Liu S, et al. (2021) J Hematol Oncol, 14: 7. [LINK NOT AVAILABLE]. Similar to the T-cell based immunotherapies, therapies based on NK cells are developed against proteins present on the surface of cancer cells which helps NK cells recognize cancer cells and kill them. However, NK cells lack many of the advantages of T cells, including the abilities to significantly expand upon recognizing their target and to form long-term memory, and don’t have the fine specificity of T cells. Currently, several NK cell-based immunotherapeutics to treat many types of cancer are at various stages of clinical development (537)Daher M, et al. (2021) Clin Transl Immunology, 10: e1274. [LINK NOT AVAILABLE].
Another group of immune cells with great potential as an immunotherapeutic are tumor-infiltrating lymphocytes (TILs). These cells are present inside the tumor microenvironment and can recognize and kill cancer cells (see Tumor Microenvironment, p. 35).
In TIL therapy, researchers remove a portion of the tumor from a patient, isolate TILs from it, grow them in large numbers quickly, and infuse them back into the patient (see Sidebar 39). In early clinical studies, TIL therapy has shown remarkable success in a number of cancer types, including melanoma and cancers of the cervix, colon and rectum, bladder, lung, and breast (539)Zhao Y, et al. (2022) Cancers (Basel), 14. [LINK NOT AVAILABLE]. As one example, cancer-free survival more than doubled in patients with advanced skin cancer who received TIL therapy, compared to those who received standard of care treatment (540)Rohaan MW, et al. (2022) N Engl J Med, 387: 2113. [LINK NOT AVAILABLE].
It is also noteworthy that, compared to the currently approved CAR-T cell-based ACT, both NK cell-based and TIL immunotherapeutics have shown greater success in treating solid tumors. An exciting new approach is to combine the power of ACTs with technological advances in genetic engineering to target specific mutations present in a patient’s tumor. There are several advantages to this approach, including personalizing the treatment to the patient’s tumor, increasing the time the engineered immune cells can stay in the body, and reducing their ability to fight a patient’s normal cells (541)Bashor CJ, et al. (2022) Nat Rev Drug Discov, 21: 655. [LINK NOT AVAILABLE]. Engineered T cells with T-cell receptors (TCRs) targeting tumors with mutations in cancer-driving genes, such as KRAS (542)Tran E, et al. (2016) N Engl J Med, 375: 2255. [LINK NOT AVAILABLE], have already shown efficacy in patients, and more trials using newly isolated TCRs specific for such targets are poised to begin in 2023 (543)Martinov T, et al. (2023) Annu Rev Cancer Biol, 7: 331. [LINK NOT AVAILABLE].
With technological advances and increased knowledge of immunology, the field of ACT is on the verge of unleashing the potential of the immune system even further for the benefit of patients with cancer.
A New Age of Therapeutic Combinations
Precision medicine approaches including immunotherapy and molecularly targeted therapy have transformed the landscape of cancer care. However, only a fraction of patients responds to these treatments, and most tumors eventually develop resistance to current treatments (see Sidebar 35) (545)Vasan N, et al. (2019) Nature, 575: 299. [LINK NOT AVAILABLE]. To address these challenges, researchers are evaluating combinations of therapeutics within and between various treatment modalities, such as immunotherapy, molecularly targeted therapy, and radiotherapy, in many clinical trials against a wide array of cancers. There is already emerging evidence that combination therapy is an effective way to overcome or delay the development of resistance (546)Jin H, et al. (2023) Nat Rev Drug Discov, 22: 213. [LINK NOT AVAILABLE].
Immunotherapeutics, especially ICIs, have shown remarkable success when used in combination with other methods of treating cancer. The success of using immunotherapeutics in combination is underscored by several FDA approvals of drug combinations to treat different types of cancer. Research has shown that the best outcomes are achieved when therapeutics are combined rationally based on their underlying mechanisms of action (547)Obenauf AC (2022) Sci Transl Med, 14: eadd0887. [LINK NOT AVAILABLE].
Among the most promising of combinations are those that combine an immunotherapeutic with a molecularly targeted therapeutic or two different immunotherapeutics (549)Zhu S, et al. (2021) J Hematol Oncol, 14: 156. [LINK NOT AVAILABLE]. Treatment with ICIs has also been effective when given before the surgery to remove the tumor (to shrink the tumor size and kill cancer cells that have spread in the body) (550)Bilusic M, et al. (2021) J Natl Cancer Inst, 113: 799. [LINK NOT AVAILABLE], or after the tumor is surgically removed (to kill remaining tumor cells) (551)Higham CE, et al. (2020) Eur J Cancer, 132: 207. [LINK NOT AVAILABLE], as well as when given in combination with chemotherapy or radiotherapy (549)Zhu S, et al. (2021) J Hematol Oncol, 14: 156. [LINK NOT AVAILABLE]. Furthermore, researchers are investigating whether combining immunotherapeutics with modulation of the patient’s microbiome (see Targeting the Microbiome in Cancer Treatment, p. 150) will further increase their effectiveness in treating cancer (552)Lu Y, et al. (2022) J Hematol Oncol, 15: 47. [LINK NOT AVAILABLE].
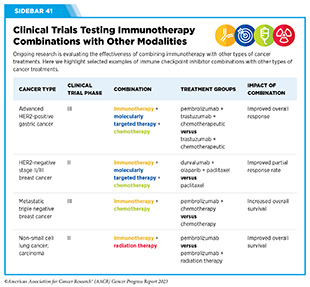
Currently, there are many therapeutic combinations being tested in clinical studies (see Sidebar 41).
Researchers are continually pioneering different methods to identify and test novel therapeutic combinations that may be more effective and safer to treat cancer, and/or may be able to overcome or delay resistance to cancer treatment. Given that the number of potential combinations of therapies is immense and will increase dramatically as the number of cancer treatments rises in the future, continued research is needed to identify biomarkers to help determine the most effective combinations. Researchers anticipate that big data approaches and machine learning technologies will further accelerate the pace of research in combination therapy for cancer. Mechanism-based rational combinations of different immunotherapeutics or with other treatment modalities have the potential to transform the future of cancer care.
Next Section: Supporting Cancer Patients and Survivors Special Section: Perspective— Looking to the Future of Immunology Previous Section: Advancing the Frontiers of Cancer Science and Medicine