Feb 04, 2025
Acclaim® Pivotal Trial
Envoy Medical recently received FDA review to begin a pivotal clinical study for a new first-of-its-kind, fully implanted cochlear implant, the Acclaim® Cochlear Implant.
CAUTION: Investigational device. Limited by federal (or United States) law to investigational use.
What are cochlear implants?
Cochlear implants provide a surgical solution for individuals with moderate to profound sensorineural hearing loss who have difficulty understanding speech even when using properly adjusted traditional hearing aids.
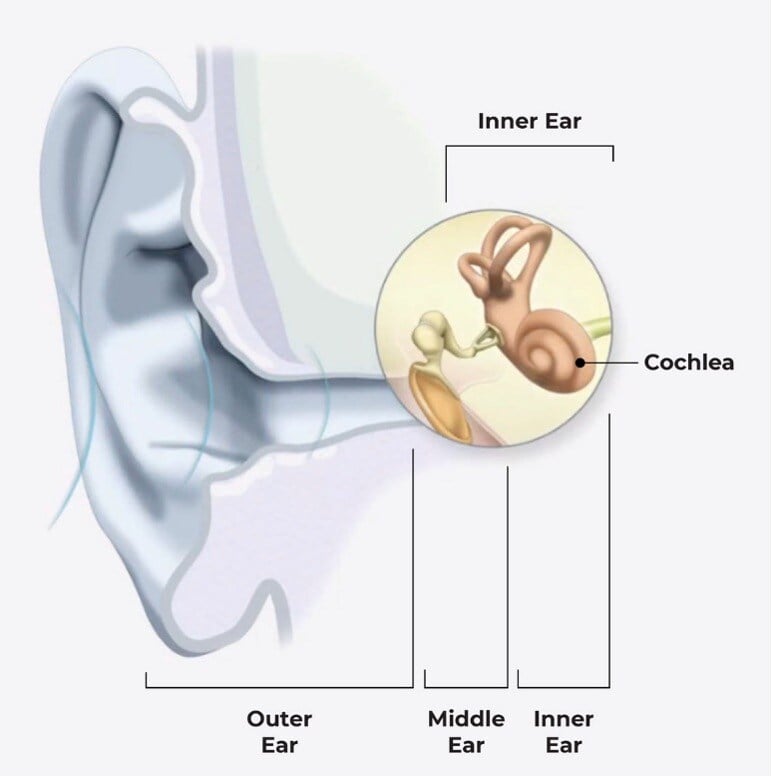
The cochlea is a small, snail-shaped organ in the inner ear, containing thousands of tiny hair cells that convert sound into electrical signals. These signals are sent to the brain, enabling us to perceive and understand sound.
In individuals with moderate to profound sensorineural hearing loss, many hair cells are damaged, and there is currently no way to regenerate or repair them.
Cochlear implants are designed to bypass damaged hair cells in the cochlea by creating a new route for sound to travel from your outer ear to your inner ear.
What makes the Acclaim® Cochlear Implant Different?
Traditional Cochlear Implant
- Partially implanted
- External components that must be worn on or behind the head for the device to work
- External microphone
- External battery
- A magnet under the skin holds the external sound processor to the head

Investigational Acclaim Cochlear Implant
- Fully implanted
- No external components are required for you to hear
- No microphone, ear canal is open
- The Acclaim battery is implanted under the skin
- Uses piezoelectric sensor technology
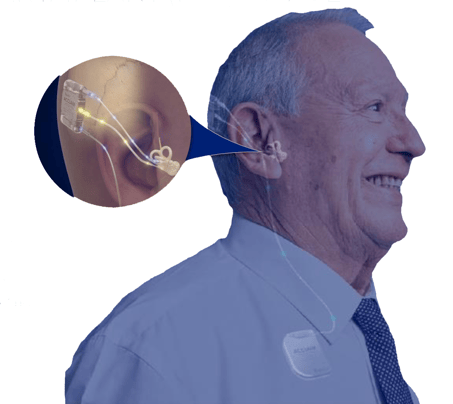
The Acclaim doesn't use a microphone like other hearing devices, it uses a special sensor called a piezoelectric sensor.
Here's how it is designed to work:
- Sound Enters the Ear: Sound comes into your ear the normal way, through the outer ear and ear canal.
- Vibrations: The sound makes tiny vibrations in a small bone in your ear called the incus.
- Sensor Detects Vibrations: The piezoelectric sensor feels these vibrations.
- Converts to Signals: The sensor changes the vibrations into electronic signals.
- Stimulates Hearing: These signals are sent to your inner ear and hearing nerve, helping you hear sounds.
The Acclaim cochlear implant uses external accessories to control and charge the internal components, but these are not required for you to hear.
The Acclaim Charger- External Battery Charger
Acclaim Charger
The Acclaim Charger has an external charger coil that is placed over the implanted Acclaim battery in the chest to recharge it.

The Acclaim charger holder enables hands-free charging by positioning the external charger coil over the implanted Acclaim battery and securing it in place once a connection is made.

Who Can Use the Device:
The device is still being tested to make sure it works well and is safe. Right now, it's only being used in a special study to learn more about it and this study has some rules for who can join. These rules are typically stricter than they will be once the device is proven to be safe and helpful and approved by the FDA. After that, more people should be able to use it.
Who Can Join the Study:
Researchers look for people who match certain rules, called eligibility criteria. These rules can include things like a person's health or past treatments.
Eligibility Criteria- Study Rules for Inclusion:
Researchers look for people who match certain rules, called eligibility criteria. These rules can include things like a person's health or past treatments.
Inclusion Criteria:
- Signed and dated consent form.
- Can understand and follow the study rules, including surgery and recovery.
- Can read, write, understand, and speak English well.
- Became deaf after learning to speak.
- 18 years old or older.
- In good health with no major health problems, according to the main researcher.
- Used hearing aids in both ears for at least 30 days.
- The ear to be implanted has very bad hearing loss (average hearing level of 70 dB or more at certain frequencies).
- The other ear has moderately bad to very bad hearing loss (average hearing level of 60 dB or more at certain frequencies).
- Limited help from hearing aids (word recognition of 40% or less in the ear to be implanted and 60% or less in the other ear, with hearing aids).
- Normal middle ear function based on ear exams.
- The ear to be implanted has a clear cochlear space and healthy cochlear nerve, with no known problems in the hearing nerve or brain's hearing pathway, confirmed by MRI or CT scan.
Who Can't Use the Device:
Some people can't use the device because of certain reasons. These reasons are called exclusion criteria. They help make sure the device is safe for the people who use it. If someone has any of these reasons, they can't be part of the study or use the device.
Exclusion Criteria:
- People who have had very bad hearing loss for 20 or more years.
- People who have had a cochlear implant in either ear before.
- People with problems in their ear bones that might stop the device from working.
- People with ear infections or other ear problems in the last 6 months.
- People with a history of eustachian tube problems.
- People who have had surgery in their ear, neck, or chest that might stop the device from working.
- People with hearing loss caused by nerve problems.
- People diagnosed with auditory neuropathy.
- People using other implants that might interfere with the device.
- People allergic to materials like silicone, rubber, stainless steel, titanium, platinum, or gold.
- People who can't take certain medicines needed for the procedure.
- Pregnant people.
- People who need an MRI or radiation treatment during the study.
- People who have unrealistic expectations about what the device can do.
- People who can't follow all the study rules.
- People with other conditions that might stop them from completing the study.
- People who were in another study in the last 3 months.
- People who had meningitis before.
- People who are deaf in the ear to be implanted because of:
- A damaged or missing eardrum
- A damaged or missing middle ear
- A missing cochlea
- Problems with the hearing nerve
- Problems with the brain's hearing pathway
This Study has 7 Locations Some Sites are Waiting for IRB Approval - Check Back for Updates
For more detailed information, go to www.clinicaltrials.gov
Arizona
Active, not recruiting
Tucson, Arizona, United States, 85718
Center for Neurosciences Ear and Hearing Center
Contact: Gwendolyn Clinical Research Nurse
(520)795-0229
Crystal Clinical Research Director
(520)529-5234
Minnesota
Open to Recruitment
Rochester, Minnesota, United States, 55902
Mayo Clinic
Contact: Nicole Tombers, RN, CCRP
Email: Tombers.Nicole@mayo.edu
Phone: 507-293-2445
South Carolina
Active, not recruiting
Charleston, South Carolina, United States, 29425
Contact: Clinical Research
CI Coordinator: 843-792-4611
email: CIProgram@musc.edu
The content is not intended to be a substitute for professional medical advice or diagnosis and should not be interpreted to contain medical advice or treatment recommendations. You should talk to your healthcare professional about what may be right for you. DO NOT disregard or delay seeking professional medical advice because of something you read or learn from this site. Envoy, Envoy Medical and Acclaim are registered trademarks of Envoy Medical Corporation.
Trending on Our Blogs
Empowering the Experts: World Hearing Day 2025
- Envoy Medical Staff Member
- March 9 2025
The Heart-Hearing Connection: Understanding the Role of Anxiety During Heart Health Month
- Envoy Medical Staff Member
- February 2 2025
New Congress 2025: The Future of the Hearing Device Coverage Clarification Act
- Envoy Medical Staff Member
- January 15 2025



