- 1York Health Economics Consortium, University of York, York, United Kingdom
- 2Cognetivity Ltd., London, United Kingdom
- 3Department of Old Age Psychiatry, South London and Maudsley NHS Foundation Trust, King’s College London, London, United Kingdom
- 4Department of Stem Cells and Developmental Biology, Cell Science Research Centre, Royan Institute for Stem Cell Biology and Technology, ACECR, Tehran, Iran
Objectives: The aim of this study was to develop a comprehensive economic evaluation of the integrated cognitive assessment (ICA) tool compared with standard cognitive tests when used for dementia screening in primary care and for initial patient triage in memory clinics.
Methods: ICA was compared with standard of care comprising a mixture of cognitive assessment tools over a lifetime horizon and employing the UK health and social care perspective. The model combined a decision tree to capture the initial outcomes of the cognitive testing with a Markov structure that estimated long-term outcomes of people with dementia. Quality of life outcomes were quantified using quality-adjusted life years (QALYs), and the economic benefits were assessed using net monetary benefit (NMB). Both costs and QALYs were discounted at 3.5% per annum and cost-effectiveness was assessed using a threshold of £20,000 per QALY gained.
Results: ICA dominated standard cognitive assessment tools in both the primary care and memory clinic settings. Introduction of the ICA tool was estimated to result in a lifetime cost saving of approximately £123 and £226 per person in primary care and memory clinics, respectively. QALY gains associated with early diagnosis were modest (0.0016 in primary care and 0.0027 in memory clinic). The net monetary benefit (NMB) of ICA introduction was estimated at £154 in primary care and £281 in the memory clinic settings.
Conclusion: Introduction of ICA as a tool to screen primary care patients for dementia and perform initial triage in memory clinics could be cost saving to the UK public health and social care payer.
Introduction
Dementia is defined as an acquired loss of cognition that affects everyday function (1). It is an umbrella term for a number of specific medical conditions, including Alzheimer’s disease (AD) (2), which is perhaps the most studied subtype of dementia. In 2019, the global number of individuals who lived with dementia was estimated at 57.4 million and, largely due to population growth and ageing, this figure is expected to approximately triple by 2050 to reach 152.8 million (3).
In the UK, 885,000 people were estimated to live with dementia in 2019; the majority of them (84.7%) residing in England (4). The number of people with dementia in the UK has been projected to increase to 1.6 million by 2040, including 1.35 million people in England alone (4). The economic burden of dementia in the country is substantial, with the total cost of care estimated at £34.7 billion across the UK in 2019, of which publicly or privately funded social care constituted 45% (£15.7 billion), informal care 40% (£13.9 billion), and health care 14% (£4.9 billion) (4). By 2040, the total costs of dementia care have been projected to rise 2.7-fold from the 2019 estimates, to reach approximately £94.1 billion (4).
Currently there are no disease-modifying therapies are available in the UK and the existing pharmacologic interventions work symptomatically (5, 6). In addition, non-pharmacological interventions such as cognitive stimulation, cognitive rehabilitation, and occupational therapy are recommended to promote independence and well-being in people with dementia (6). However, there are still potential benefits to diagnosing dementia early. Firstly, there is some evidence beginning to emerge that early treatment for AD delays cognitive decline (7). Secondly, early awareness of diagnosis facilitates informed decisions, e.g., related to financial and legal planning or future care needs (8). Finally, with the advent of disease-modifying therapies (DMTs), which target the pathophysiology of AD to delay its progression (9), the importance of early dementia diagnosis will be growing in years to come and early diagnosis may provide the opportunity to participate in research.
Early diagnosis of dementia at a stage where the cognitive impairment is still mild can be achieved in the primary care setting using an established cognitive assessment instrument (8, 10). In the UK, individuals with suspected dementia are subsequently referred to a memory clinic to establish the subtype of dementia based on further diagnostic testing and initiate therapy as appropriate (10). Cognitive testing is often also employed during initial triage in the memory clinic (11). The COVID-19 pandemic, however, necessitated conducting cognitive assessment remotely, despite unclear validity and reliability of such assessments using currently available tools (12).
The integrated cognitive assessment (ICA), trademarked as CognICA™, is a brief, language independent, self-administered, computerized cognitive test, which uses an explainable artificial intelligence model to improve its accuracy of cognitive impairment diagnosis (13–15).
The ICA is previously validated across multiple studies as an accurate cognitive assessment tool compared with clinical diagnosis (13–18) and have further demonstrated convergent validity with existing standard of care tests, such as MoCA and ACE (13–15). The tool is also CE-Marked1 and FDA-registered2 as a software-as-medical-device.
The ICA aims to facilitate and streamline dementia diagnosis in the National Health Service (NHS). In this real-world Accelerating Dementia Pathway Technologies (ADePT) study (19), the objective was to assess health economic benefits of this novel clinically validate ICA test in comparison to the current standard of care (11). Therefore the current analysis offer a comprehensive economic evaluation of the ICA tool compared with standard cognitive tests used for dementia screening in the primary care setting and for initial patient triage in the memory clinic setting.
Methods
In the base case analysis, the ICA tool was compared to standard cognitive testing in the primary care setting, comprising the mini-mental state examination (MMSE), general practitioner assessment of cognition (GPCOG), the six-item cognitive impairment test (6CIT), the abbreviated mental test score (AMTS), the Montreal cognitive assessment (MoCA) and a small proportion received a mix of other tests. A scenario analysis was also conducted in people with suspected dementia that have been referred to a memory clinic, where the ICA tool was tested against standard care comprising the Addenbrooke’s cognitive examination-III (ACE-III) and the Montreal cognitive assessment (MoCA). Quality of life outcomes were quantified using quality-adjusted life years (QALYs). Both costs and QALYs were discounted at 3.5% per annum in line with National Institute for Health and Care Excellence (NICE) guidance (20). The NICE reimbursement threshold of £20,000 per QALY gained was used to assess the cost-effectiveness of the ICA tool over a lifetime horizon.
Ethics approval
Health Research Authority and Health and Care Research Wales approval for this study was obtained in February 2020. The study is registered in the ISRCTN Registry (ISRCTN16596456). Approved 27/02/2020, North of Scotland Research Ethics Committee (Summerfield House, 2 Eday Road, Aberdeen, AB15 6RE, UK; +44 (0)1224 558458; nosres@nhs.net), ref.: 20/NS/0029.
Model structure
Model structure is summarized in Figure 1. The structure was informed by the Sussex partnership memory clinic care pathway and a targeted literature review. The model employed a decision tree to capture the initial outcomes of the cognitive testing, followed by a Markov structure to capture long-term outcomes after the initial test. A 1 year cycle length was used to capture dementia progression in the Markov model. The model includes several states such as mild cognitive impairment (MCI), diagnosed dementia, undiagnosed dementia, and Other/Healthy, along with the associated transitions from mild to moderate to severe and dead. Please refer to Figure 1 for a graphical representation of the model’s structure. The perspective of the analysis was that of the UK NHS and Personal Social Services (PSS).
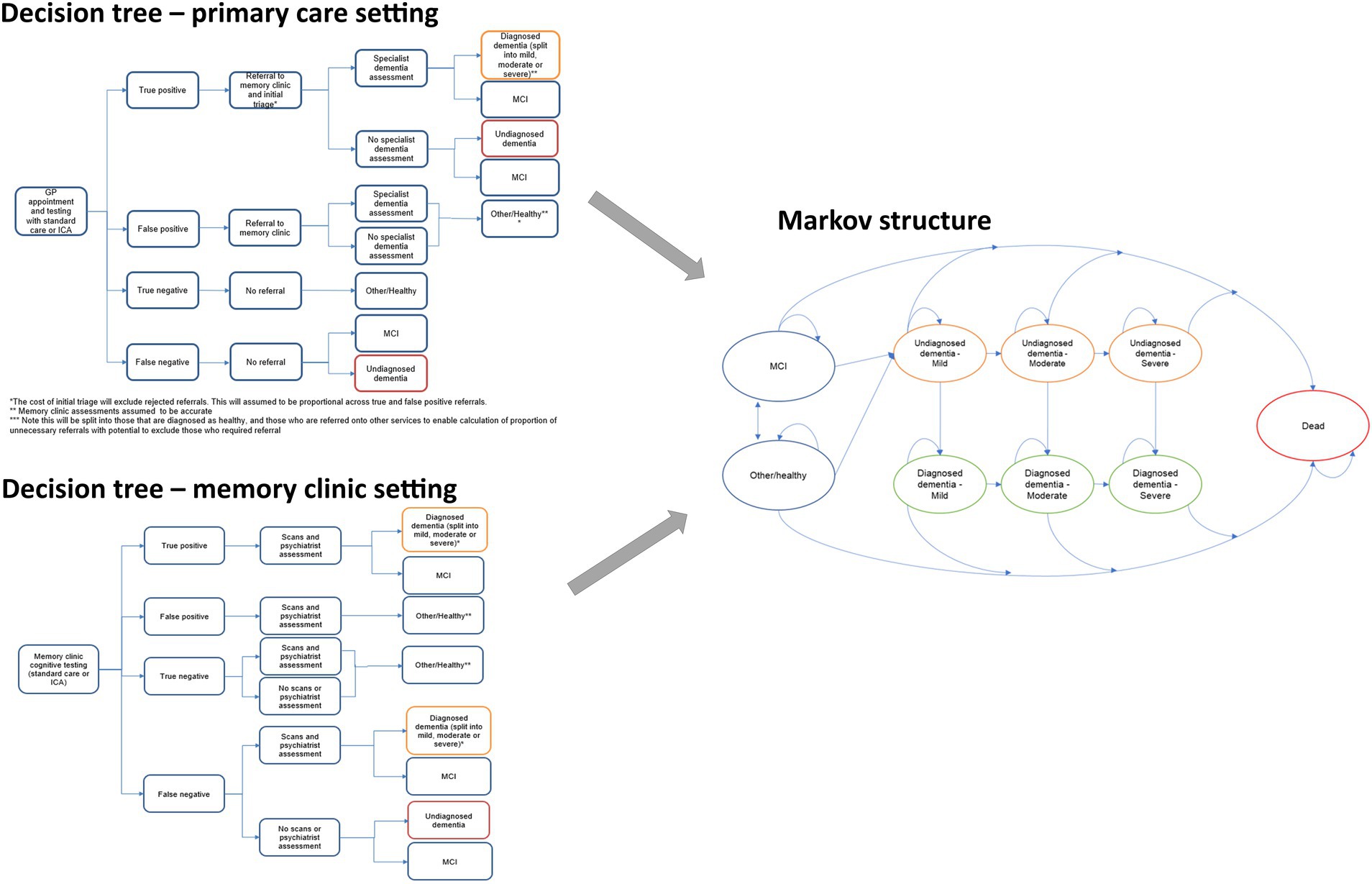
Figure 1. Structure of the model including the two alternative decision tree components (left) and the Markov component (right).
Clinical and quality of life inputs
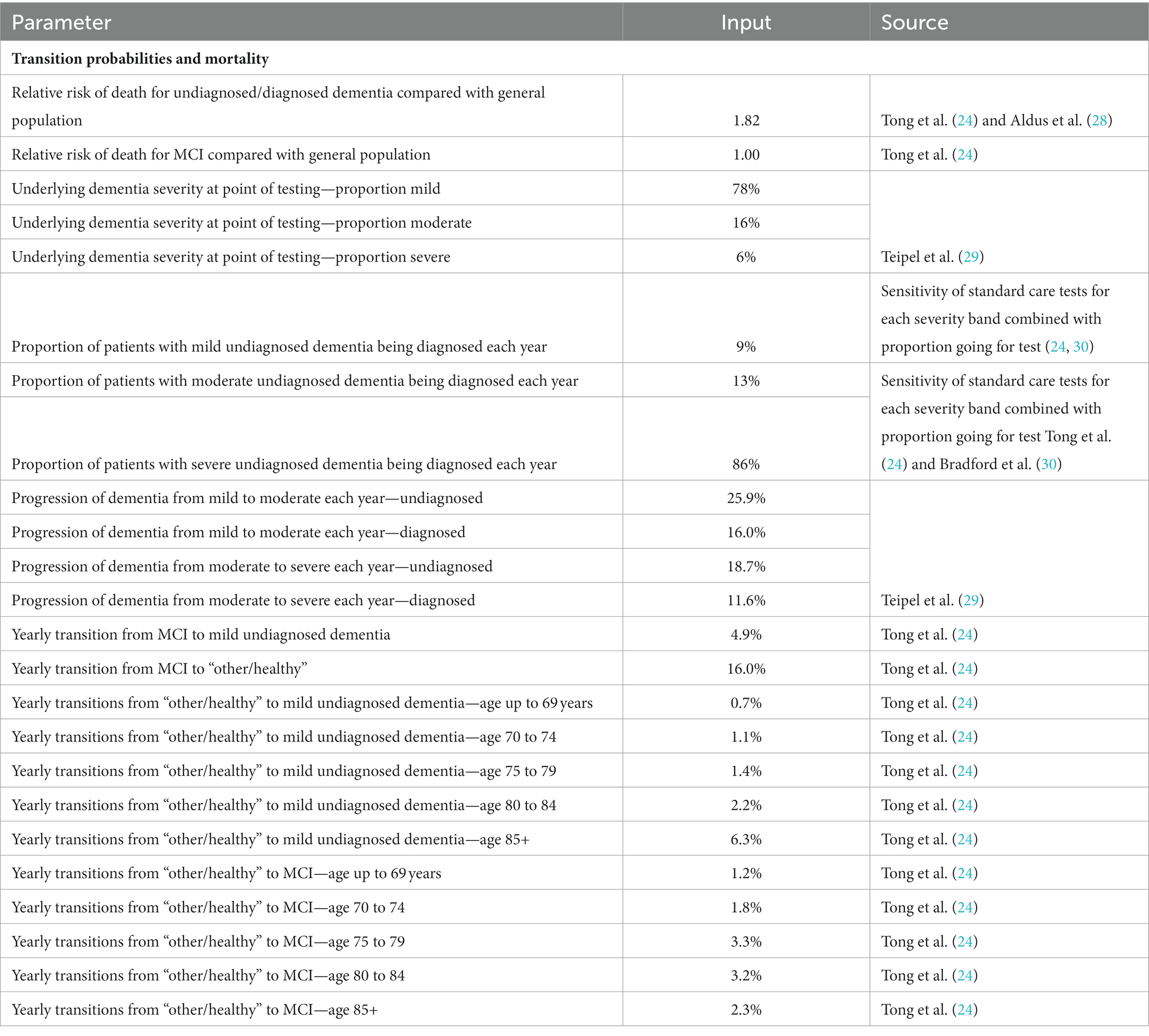
A summary of model inputs is provided in Tables 1–7.
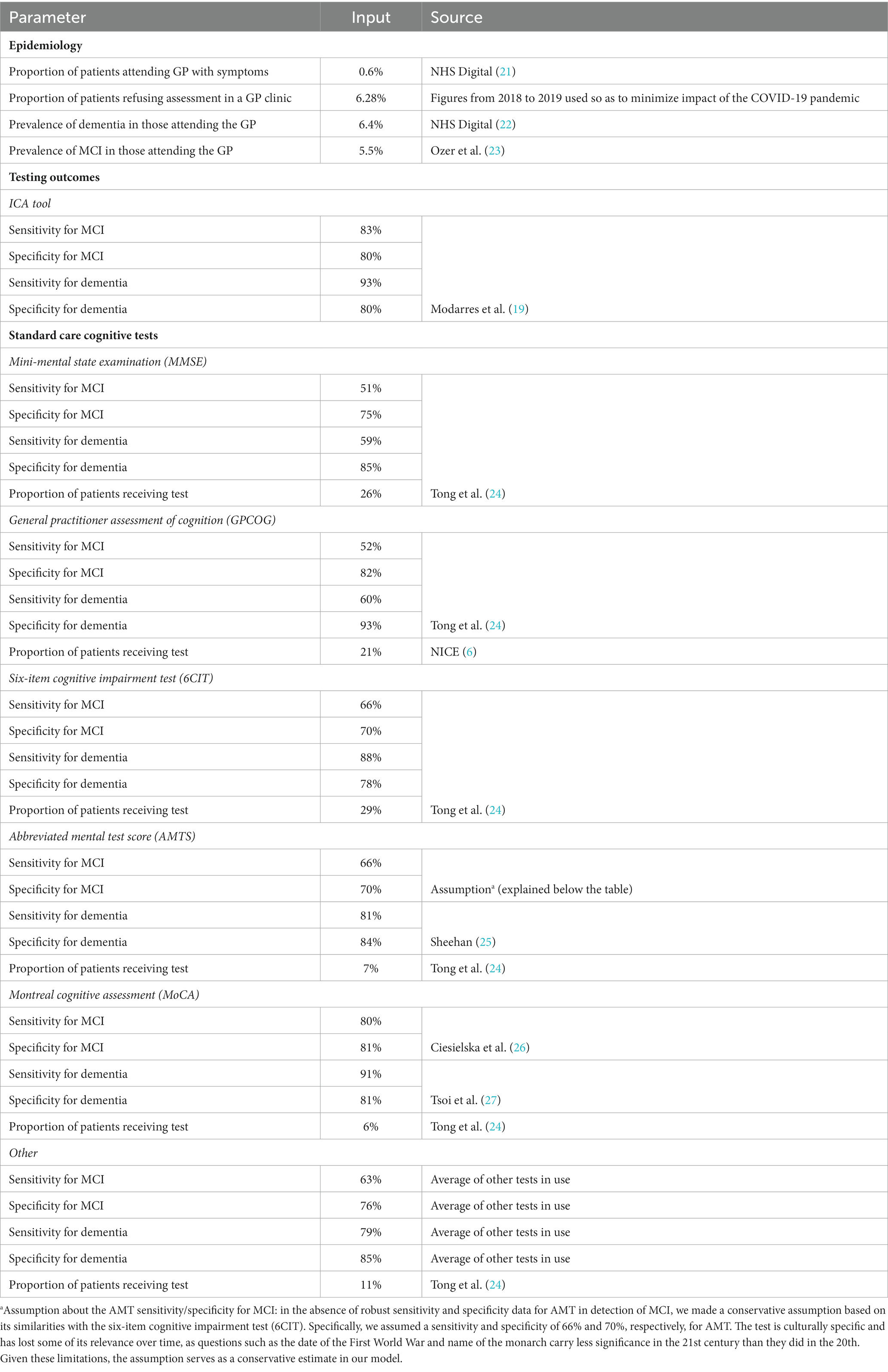
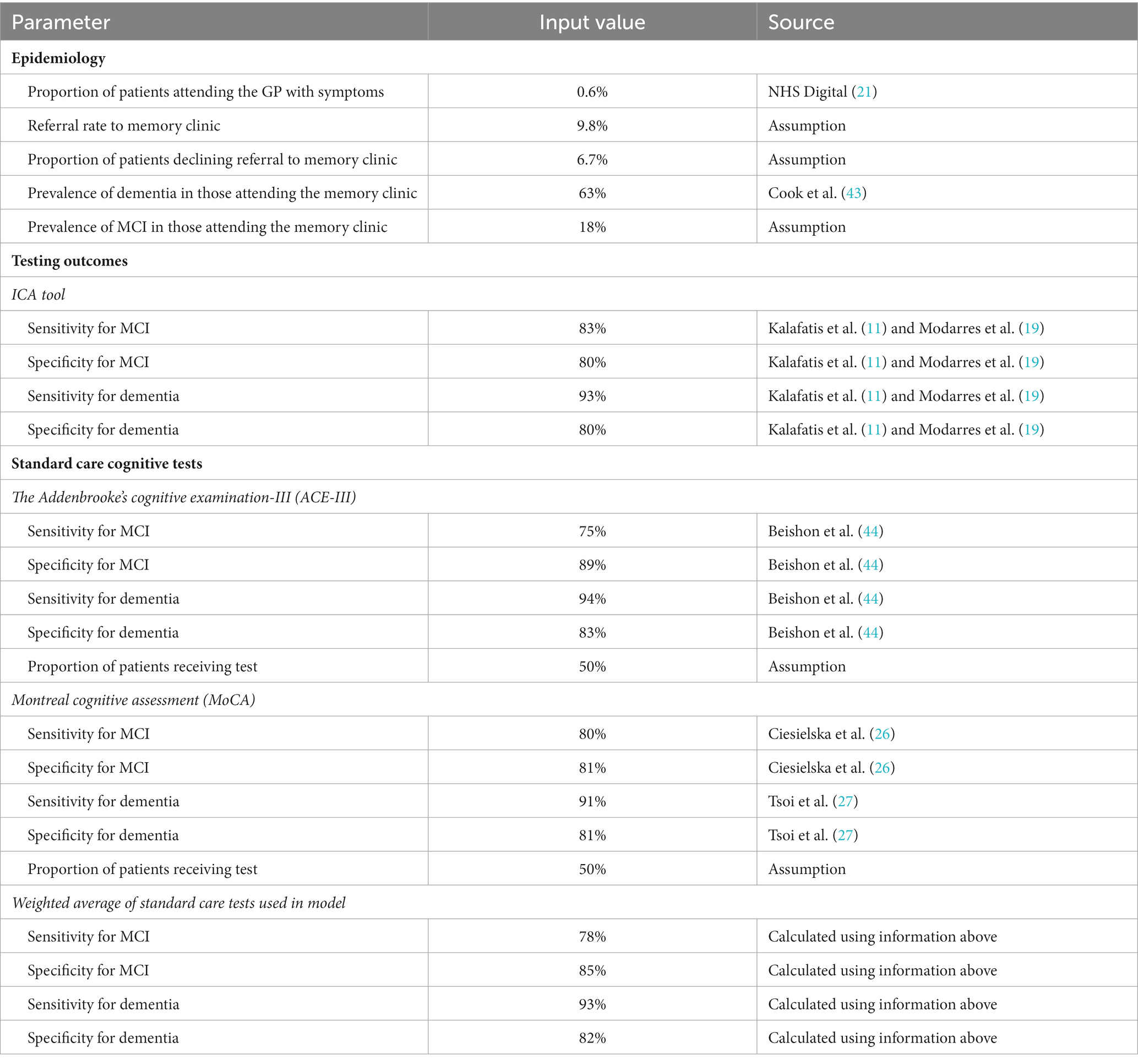
Table 1. Model inputs applied in the base case and scenario analyses: base case (primary care setting).
Sensitivity and specificity of the ICA tool for diagnosing mild cognitive impairment (MCI) and dementia were derived from the results of the ADePT study (11, 19). The results from this study informed the performance of the ICA tool in comparison with specialist diagnosis obtained at the memory clinic, i.e., the accuracy of ICA for referral of patients to a memory clinic.
Underlying dementia severity in year 1 (i.e., at the point of testing) was based on the Teipel et al. (29) model assessing the cost-effectiveness of donepezil for AD and was assumed to be the same regardless of diagnosis status (i.e., in people with dementia who are diagnosed vs. undiagnosed). The proportions of the modelled population that were diagnosed with mild, moderate, and severe dementia in subsequent years were taken from the literature and assumed to be equal between the two testing arms. Progression of undiagnosed and diagnosed dementia was derived from Teipel et al. (29) Transition probabilities from MCI to mild undiagnosed dementia and between the “other/healthy” health state and MCI were sourced from a study by Tong et al. (24) assessing the cost-effectiveness of different cognitive screening tests for use in the primary care setting.
Mortality was modelled using UK general population data for individuals in the “healthy/other” health state and relative risks were applied for people with dementia to capture increased risk of death. The relative risk of death with dementia was assumed to be the same regardless of whether the condition was diagnosed or undiagnosed, in line with a previous study (28). People with MCI were assumed to die at the same rate as the general population, based on the study by Tong et al. (24).
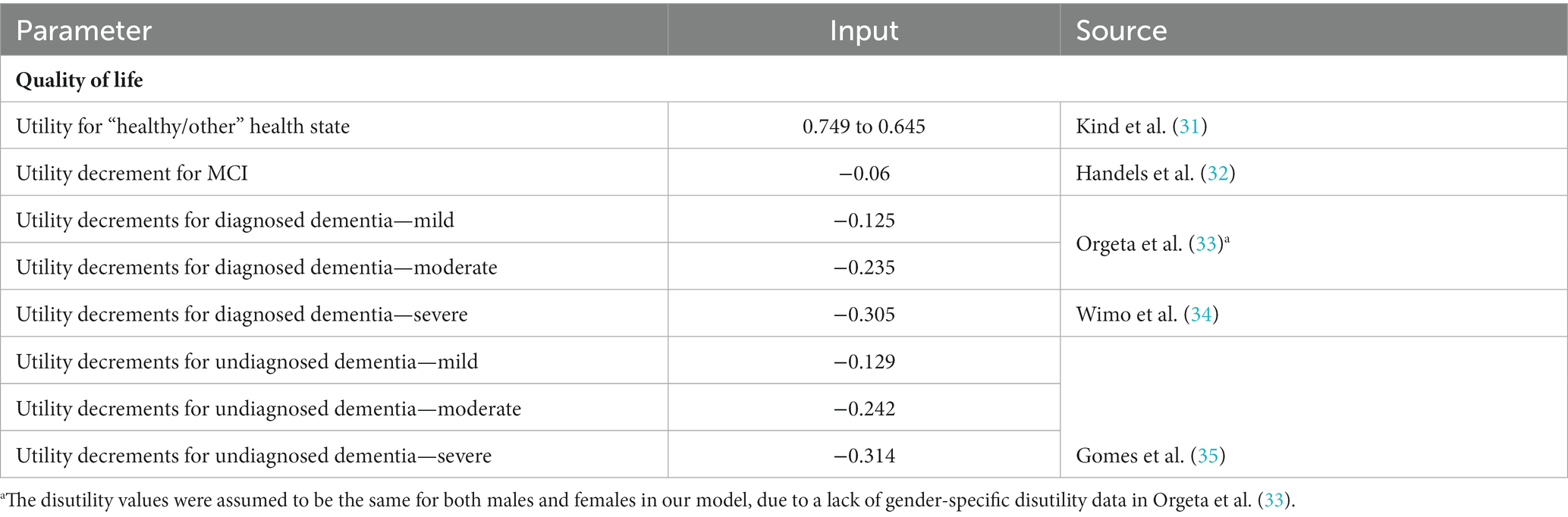
Utility values were estimated using UK population norms (31) with literature-derived decrements applied to dementia health states.
In our Markov model, mortality risks are specifically tailored to the age and health state of the individuals in the cohort. Mortality rates are derived for the general population, those with mild cognitive impairment (MCI), undiagnosed dementia, and diagnosed dementia. These rates are adjusted according to the age of the cohort, starting from 77 years and extending onwards. This allows the model to capture the nuanced mortality risks associated with both the progression of cognitive decline and aging. For example, the death rate for the general population at age 77 is 3.5%, whereas for those with diagnosed or undiagnosed dementia, it is 6%. These rates change as the cohort ages, reflecting the increased mortality risk associated with older age and the progression of the disease. This nuanced approach ensures a more accurate representation of long-term outcomes.
Cost and resource use inputs
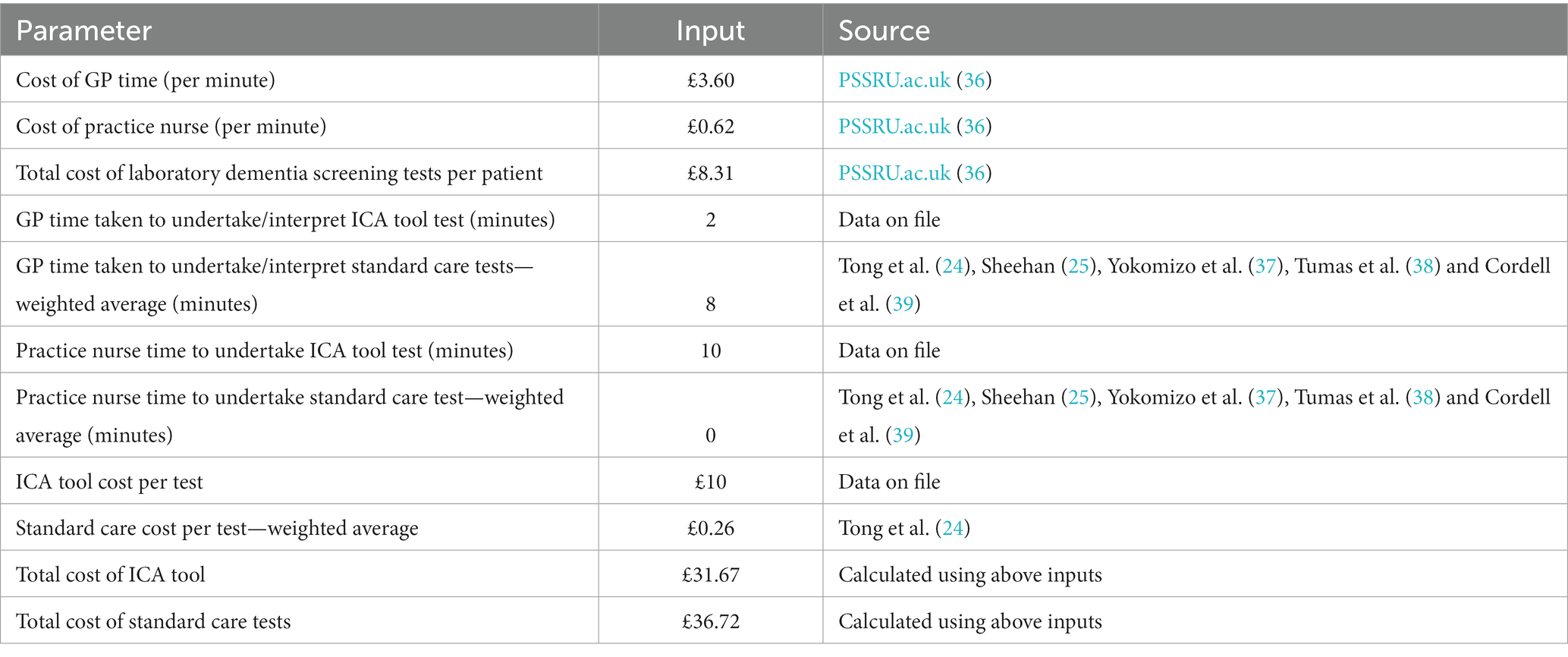
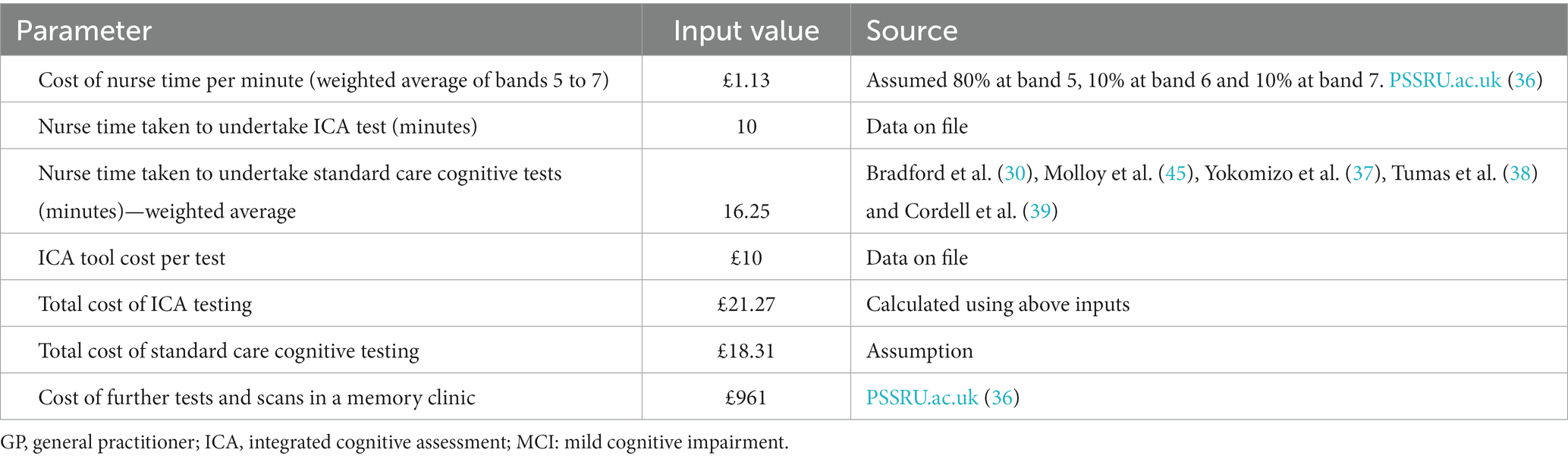
The model was populated using epidemiological and cost and resource use data from official NHS and PSS data sources, wherever available (cost year 2019). Costs of testing included in the model comprised staff time to conduct the cognitive tests, laboratory testing, and the costs of further assessment and scans at a memory clinic, in addition to the cost of the ICA. The costs were not inflated to the current year of the analysis/publication, which may affect the accuracy of the economic evaluations. This is a limitation that will be considered in future updates to the model.
The cost of ICA comprised a one-off implementation fee of £2,000, followed by a minimum monthly fee of £1,000 for 100 tests with each test thereafter charged at £10. Implementation fees were charged per trust and annuitised in the model. Sourcing of iPads or tablets, which are required for ICA to operate, were not included assuming these would be already available at the primary care practice or memory clinic settings. Staff time for training on the use if the ICA tool was also not included in the model.
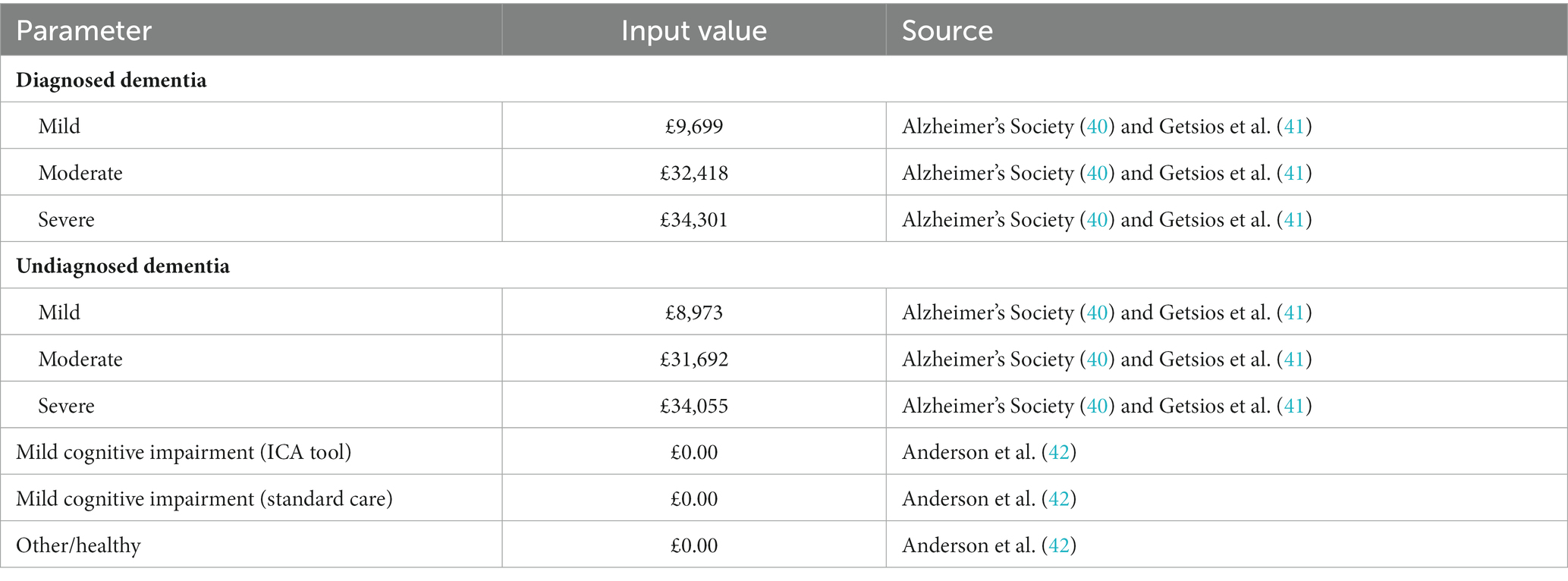
Based on the methods used by Getsios et al. (41), the costs of undiagnosed and diagnosed dementia were assumed to be the same, except for the cost of treatment which was applied only to those who had been diagnosed. This assumption can be considered conservative, since potential cost savings associated with earlier diagnosis (e.g., due to delay in admission to care homes) have been reported in the literature (46, 47). Treatment costs were based on NICE guidelines and costed using the BNF (10, 48).
Model outputs
The following outcomes were assessed for each testing arm of the model and compared between arms: total costs per person, total QALYs per person, total number of diagnoses per modelled population, total number of unnecessary referrals per modelled population, and total number of scans/assessments at a memory clinic per modelled population. Head-to-head incremental comparison between the ICA tool and standard care was based on the incremental cost-effectiveness ratio (ICER), the incremental net health benefit (NHB); and the incremental net monetary benefit (NMB).
Sensitivity analysis
In order to account for uncertainty around the input parameter values, one-way deterministic sensitivity analysis (DSA) was included in the model. The ranges used to vary the parameters were based on confidence intervals, or assumptions were made where these were not available. A probabilistic sensitivity analysis (PSA) was also implemented, whereby, depending on the parameter of interest, Dirichlet, gamma, lognormal or beta distributions were fitted to uncertain parameters to generate the input values for each iteration. The standard errors used to generate probabilistic values were derived from reported 95% confidence intervals wherever possible. Where this information was not available (e.g., for some costs), the standard error was assumed to be equal to 25% of the mean value. The appropriate number of iterations for PSA was determined by observing the number of iterations required for the average results to stabilize.
Scenario analysis
For the sensitivity and specificity of ICA used for initial triage in the memory clinic, the tool was assessed in a scenario analysis assuming equivalent sensitivity and specificity as in the base case analysis, except when real-world data from deployments of the ICA in memory clinics were available. Model inputs specific to this scenario are listed in Tables 1–7 below the base case inputs.
Two additional scenarios are presented in the Supplementary material: a scenario considering the use of the ICA tool for remote initial assessment of patients with symptoms of dementia and a scenario considering the use of the ICA tool for remote monitoring of MCI patients.
Results
Base case model results (primary care setting)
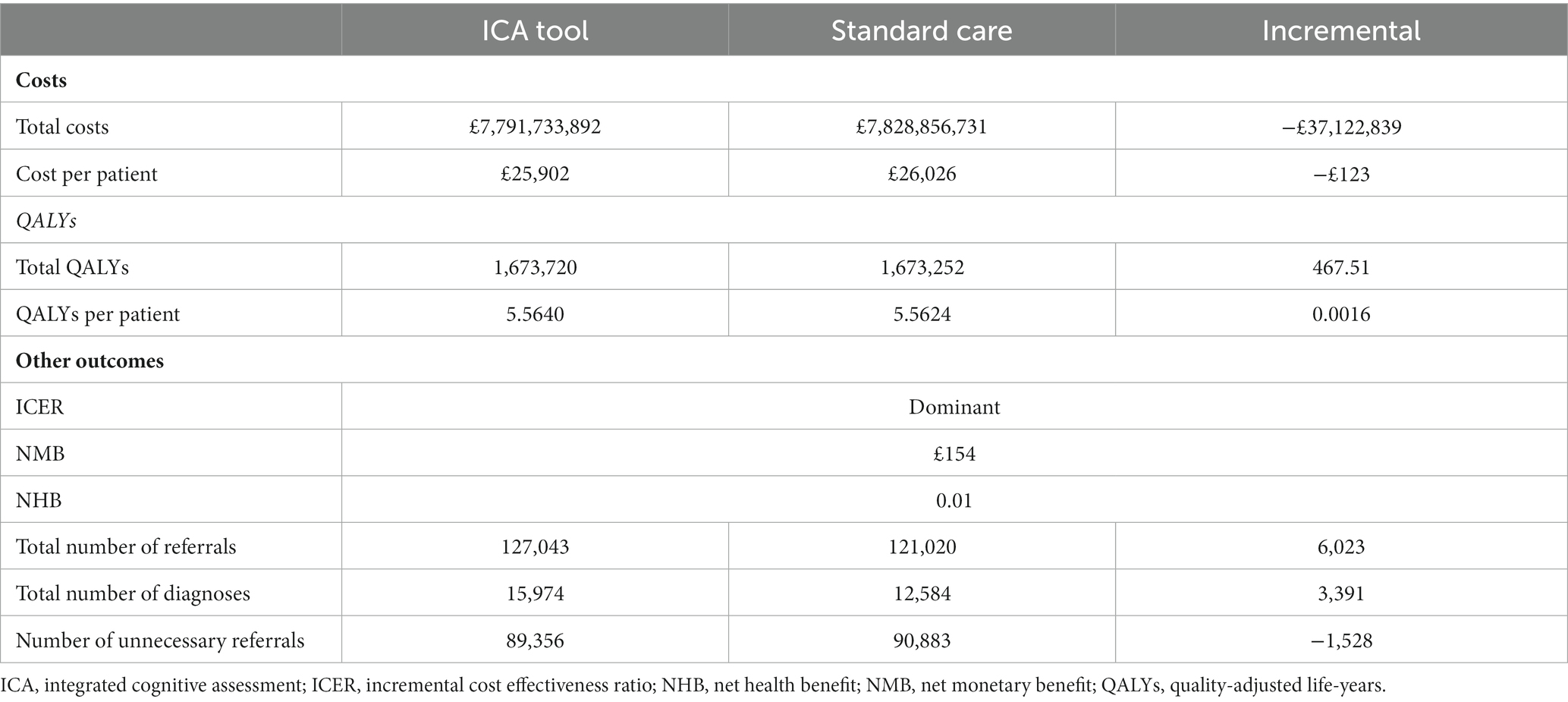
The ICA tool dominated standard care comprising currently used cognitive tests, with an estimated NMB of £154 per person (Table 8). The use of the ICA tool in the primary care setting to facilitate preliminary diagnosis of dementia and referral to memory clinics resulted in cost savings of around £123 per person over a lifetime time horizon. This equated to a cost saving to the NHS of approximately −£37,122,839. when considering the total population in a given year who may benefit from the introduction of the ICA tool. A small incremental QALY benefit of 0.0016 per person was also estimated as a result of earlier dementia diagnosis.
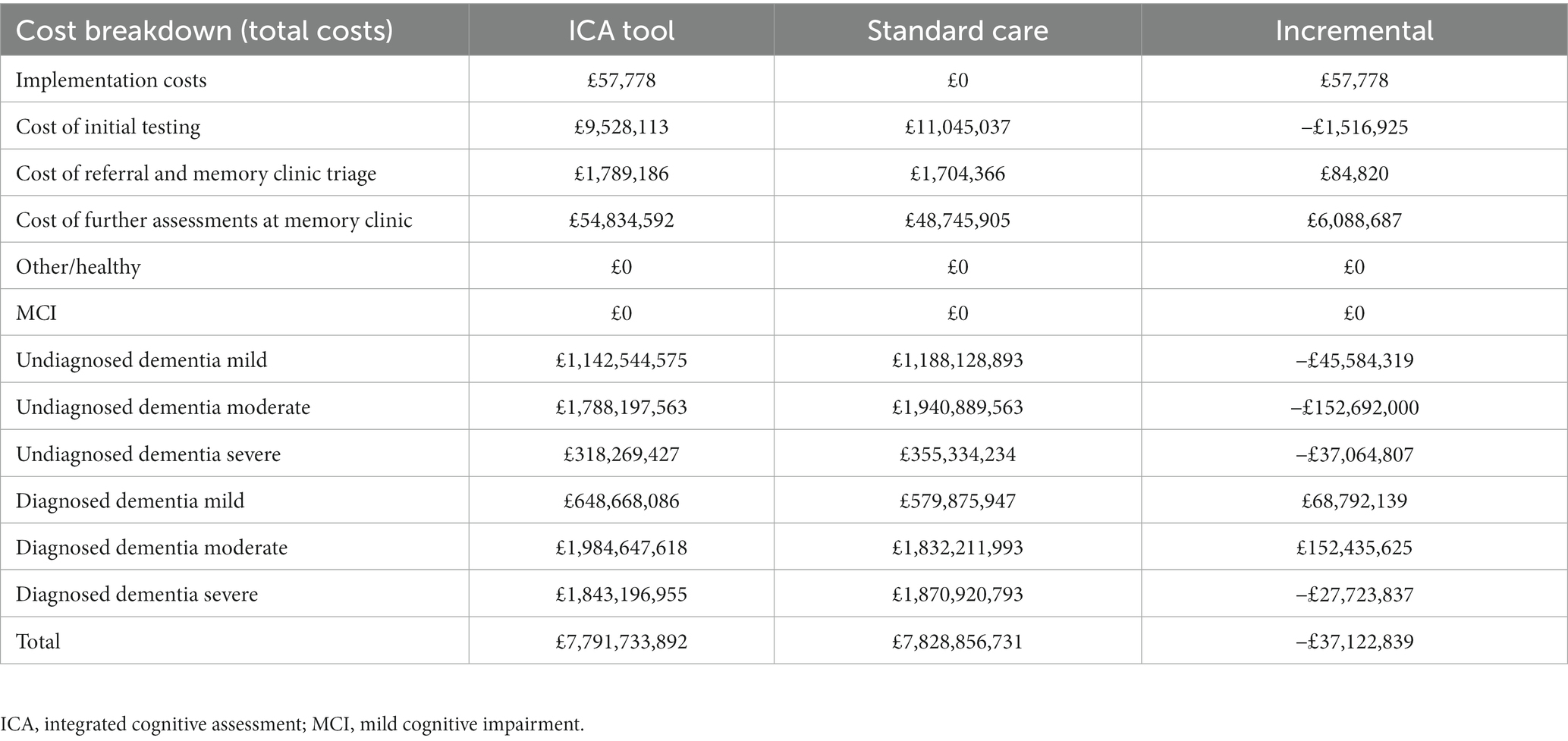
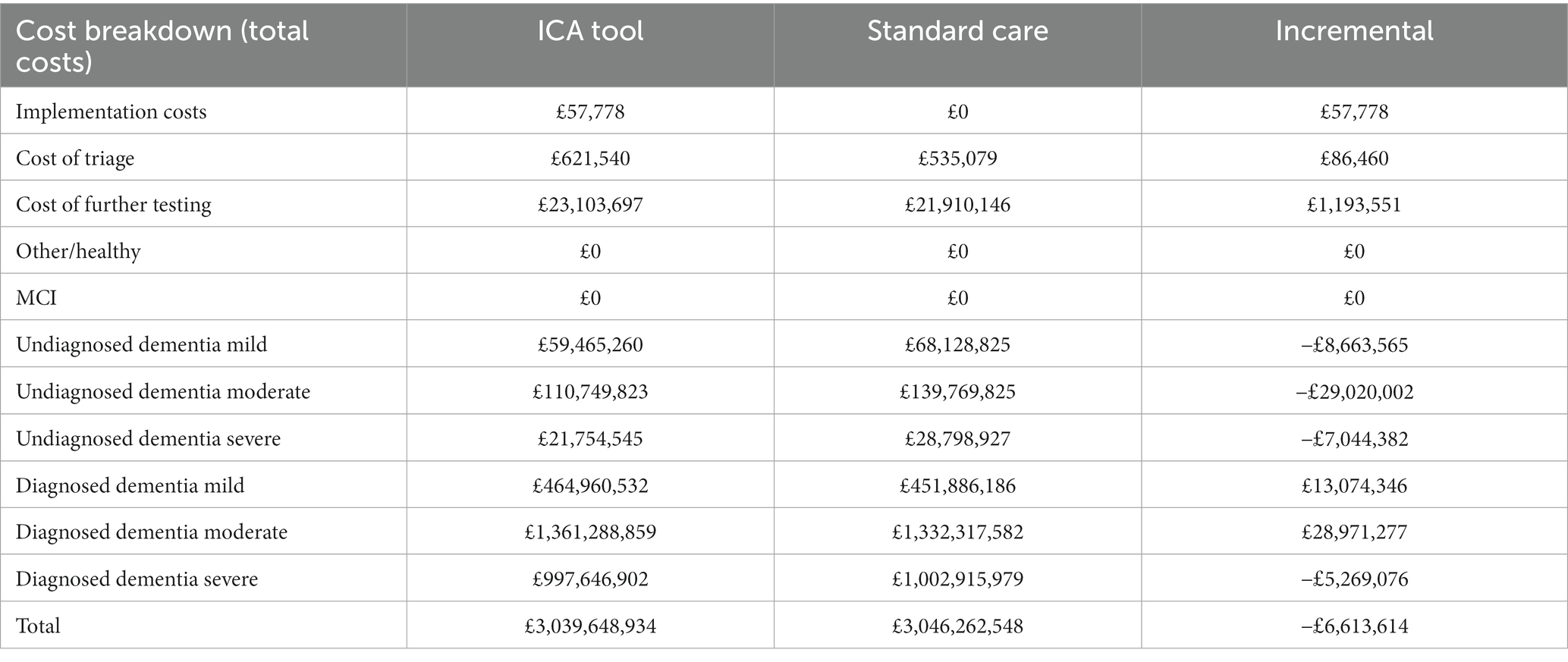
Although there were additional costs associated with introducing the tool (implementation costs and test fees for ICA and increased costs to memory clinics for increased referrals), these costs were outweighed by the reduction in staff costs associated with conducting the initial cognitive tests and a reduction in dementia care costs associated with earlier diagnosis and therefore delayed progression to more severe dementia states (Table 9).
Sensitivity analysis
Detailed results of the sensitivity analysis are presented in the Supplementary Figure S1. Key cost-effectiveness drivers included the progression of undiagnosed dementia (transition from undiagnosed mild to undiagnosed moderate dementia), and the relative risk of death for people with undiagnosed dementia vs. the general population. Each of these parameters could change the direction of the results when varied alone. PSA results indicated that the probability of the ICA tool being cost-effective was 72% at the £20,000 per QALY threshold [see Supplementary Figure S3 for the cost-effectiveness acceptability curve (CEAC)].
Scenario analysis (memory clinic setting)
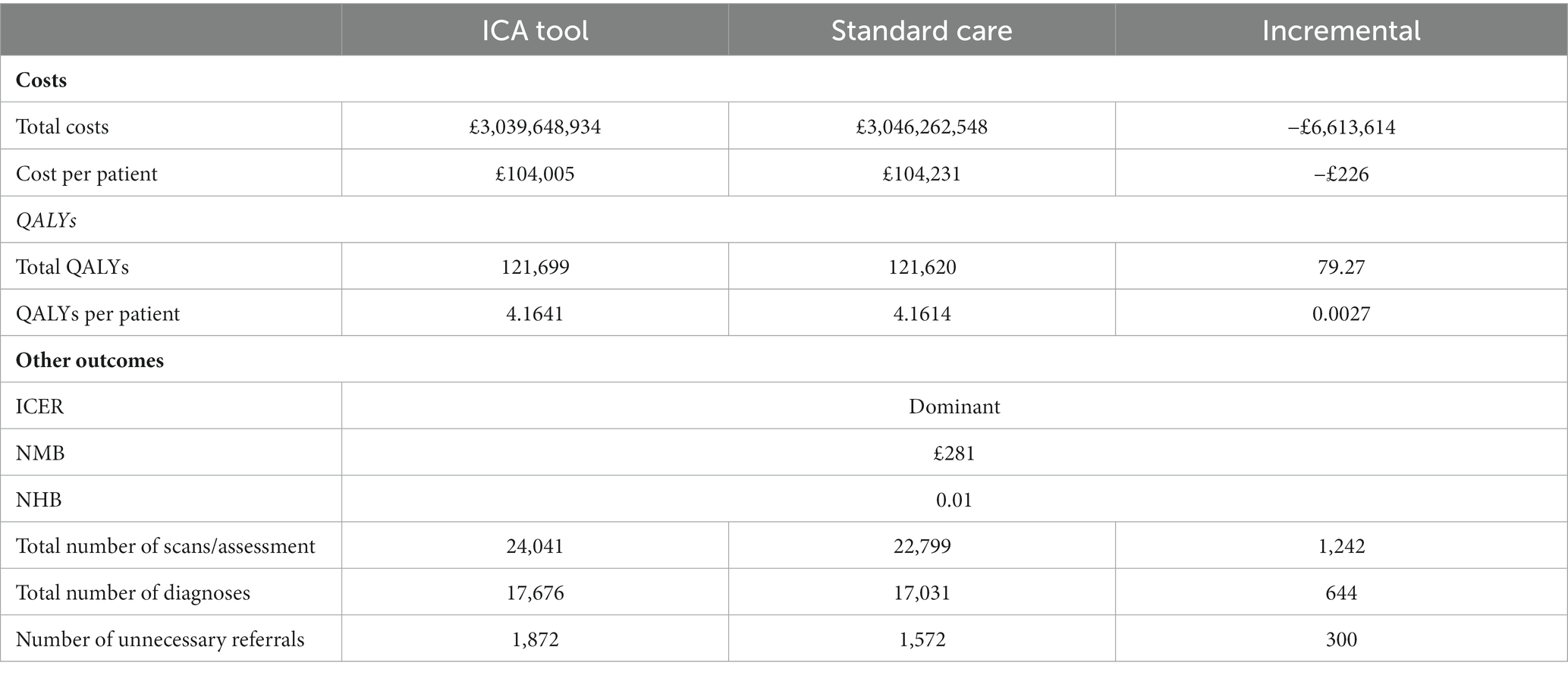
When introduced as a triage tool in memory clinic services, ICA dominated standard care and NMB was estimated at £281 per person. Employing the ICA tool saved approximately £226 per person over a lifetime time horizon, which equated to an overall cost saving to the NHS of around −£6,613,614 (Table 10). Similar to the primary care setting, there was a small QALY gain of 0.0027 per person associated with earlier diagnosis of dementia.
The increase in initial costs due to the increased number of individuals being tested for dementia was outweighed by savings due to diagnosing dementia earlier and therefore delaying progression to more severe states of the disease (Table 11). The costs in the memory clinic setting were much higher compared with the primary care setting, due to substantially higher prevalence of dementia in this setting.
DSA results revealed that the most influential parameter was the sensitivity of the ICA tool for diagnosing dementia in the memory clinic setting, which was varied by an arbitrary range of ±20% pending availability of the relevant data from the ADePT study. This parameter, alongside the two parameters already described as influential in the base case analysis (transition probability from undiagnosed mild dementia to undiagnosed moderate dementia and the relative risk of death for people with undiagnosed dementia vs. the general population) could alter the direction of the results when each of them was varied alone. In terms of PSA results, the probability of the ICA tool being cost-effective in the memory clinic setting was 63% at the £20,000 per QALY threshold [see Supplementary Figure S4 for the cost-effectiveness acceptability curve (CEAC)].
Discussion
The ICA tool dominated cognitive assessment tests currently used in both the primary care and memory clinic settings. Introduction of the ICA tool was estimated to result in a lifetime cost saving to the NHS of approximately £123 per person undergoing cognitive assessment in the primary care setting and £226 per person being triaged in the memory clinic setting. Although the model predicted an increase in costs from increased referrals and specialist assessments, these were outweighed by a reduction in the staff costs associated with initial cognitive testing and a reduction in the costs of treatment and care for people with dementia achieved through earlier diagnosis. Increasing referrals and improving diagnosis rates is in line with the Department of Health national dementia strategy (49).
The potential for earlier diagnosis of dementia through the use of the ICA tool is particularly important given that the first DMTs for AD were approved in 2021 and 2023 (i.e., Aduhelm and Leqembi) by the Food and Drug Administration (FDA) (9). Furthermore, the AD pipeline appears rich with other DMT candidates likely to also receive approvals and becoming available (50). Potential introduction of cost-effective DMTs for AD and other types of dementia would further improve the case for early diagnosis of the condition and therefore increase the cost-effectiveness of more accurate screening (51, 52) using tools such as the ICA. The progression of dementia for those who have mild undiagnosed dementia in relation to those who have been diagnosed (i.e., they are assumed to be receiving treatment) is a key driver in the model and could change the direction of the results if there is no difference in progression of dementia with treatment. Therefore, further research in this area will also improve the robustness of the model results.
The results from the present study align reasonably well with previous UK-based analyses of similar decision problems published in recent years. Tong et al. (24) reported on a cost-effectiveness analysis undertaken to assess the use of different cognitive screening tests for diagnosing dementia and MCI in primary care. The results of Tong et al. (24) employing the health and social care perspective were similar to this analysis, ranging from a cost of £6.93 to a saving of £185 per person over a lifetime horizon, depending on the cognitive test used. However, the incremental QALYs reported were more optimistic than in the present analysis (24), potentially due to the differences in sources informing utility data. Getsios et al. (41) conducted an economic evaluation comparing early assessment and treatment for AD with early assessment and no treatment, as well as treatment without early assessment. Although the estimated cost savings from early assessment and treatment were much higher than that in this analysis (£2,135 per person when compared to treatment without early assessment and £3,593 when compared to no early assessment and no treatment) (41), some important differences in model design and assumptions between the Getsios et al. model and the present study could explain these differences. The Getsios et al. analysis estimated the potential savings from assessing and treating all individuals with dementia early and assumed 100% assessment accuracy, whereas this study estimated cost savings resulting from only a proportion of individuals benefiting from earlier diagnosis and treatment, i.e., those diagnosed with the ICA tool that would have not been diagnosed with standard cognitive tests. Therefore, the cost savings estimated in this analysis could be expected to be more modest.
Although the present model concerns a highly relevant issue considering the ageing UK population and the potential advent of DMTs for dementia, it should be noted that the analysis was subject to several limitations. Firstly, the ADePT study which informed inputs related to ICA accuracy did not include a comparison between ICA and other cognitive assessment instruments, although it did compare the outputs from the ICA tool with further testing undertaken in a memory clinic which can be considered the gold standard. Since the sensitivity and specificity inputs for standard cognitive tests were based on information extracted from the literature with no attempts to validate them, the current study should be considered a naïve comparison. Additionally, the ADePT study did not include participants who were not referred by their GPs to memory clinics, so its population is somewhat different than the population included in the model.
There is a paucity of data around the influence of diagnosis on the health care costs and quality of life associated with dementia. Therefore, the health care costs associated with diagnosed and undiagnosed dementia for each severity level were assumed to be the same, except for treatment costs assigned only to individuals who had received a dementia diagnosis. The potential benefits associated with earlier diagnosis of dementia have been described in the literature (8), however, no studies generalizable to the UK NHS that quantify these benefits in terms of the relative difference in health care costs between individuals with and without a diagnosis could be identified. Similarly, the quality-of-life benefits associated with diagnosis do not seem to be widely reported across prior cost-effectiveness analyses and literature reviews (41, 46, 47, 53). The base case utility value employed in the present study was based on Gomes et al. (35), who reported EQ-5D data at baseline and 6 months following diagnosis. However, 6 months after diagnosis can be considered a short time frame in a chronic condition, so employing this value would lead the model to understate quality of life benefits if quality of life gains were made past the first 6 months following diagnosis. The uncertainty associated with the cost and quality of life impact of dementia diagnosis could affect the cost-effectiveness of the ICA tool, as there were fewer individuals with undiagnosed dementia in the ICA arm of the model, so that increasing the cost or disutility applied to undiagnosed dementia would improve the cost-effectiveness of the ICA tool, while reducing these costs or utility decrements would decrease the cost-effectiveness of the tool.
The model also did not account for all potential costs. The cost of supplying iPads or tablets, which would be required for the ICA tool to be used in practice, was not included, assuming that these would be available at GP practices and/or memory clinics. In reality, most clinics and practices can be expected to have access to such devices, so that the costs of any additional purchases would likely be negligible. In addition, no costs were assigned to individuals in the MCI health states who were assumed to receive no regular monitoring or treatment in line with current treatment pathways. If such costs were included, they would be higher in the ICA tool arm of the model, so any benefits associated with monitoring would need to outweigh these additional costs.
The model employed the NHS and PSS perspective; consequently, the costs associated with informal care and other out-of-pocket costs were not included, even though much of the cost and burden of care falls on the person with dementia and their family (54). Anderson et al. (42) estimated that the costs of unpaid care and other costs falling on individuals vary between £9,711 for individuals with severe dementia to £17,917 for those with milder dementia. This equates to anywhere between approximately 25% of the total costs estimated per year for individuals with moderate and severe dementia up to 70% for those with milder dementia who may receive less formal care (42). However, there is substantial uncertainty associated with costing informal care. The aforementioned analysis by Tong et al. (24) estimated only a small change in results when informal care costs were included. It should also be noted that, in addition to informal care costs, the costs of lost productivity (for people with dementia or their caregivers) were also not considered, even though by 2040 these costs are estimated to reach £1.3 billion to businesses in England (40). If informal care costs and productivity costs were to be included in this analysis this would further improve the cost-effectiveness of the ICA tool.
Regarding the generalizability of the model, it is also worth noting that our model focuses on a specific setting and cohort. While the results provide valuable insights for that particular context, caution should be exercised when generalizing these findings to other settings or populations. We acknowledge the importance of using large and representative real-world data sets to inform and validate health-economic models. Our model incorporates data from only one study [i.e., Modarres et al. (19)], which may limit the generalizability of our findings.
Quality of life of caregivers or family members was also not considered, despite the substantial impact that caring for people with dementia has on them (55). Including these costs and quality of life benefits would likely improve the case for the adoption of the ICA tool because higher costs associated with dementia, particularly more severe states, could be expected to result in higher cost savings (provided the costs for diagnosed individuals are not substantially higher than those for undiagnosed individuals). It should be noted, however, that the cost-effectiveness threshold used in this analysis would no longer be relevant when considering the societal perspective because it is intended to represent the opportunity cost of funding an intervention using NHS and PSS budgets. Therefore, interpretation of the cost-effectiveness results is more difficult when considering this wider perspective.
A further limitation of the model is that it does not consider the wider impact of introducing the ICA tool on health care system capacity. Introducing a cognitive test that has increased sensitivity but decreased specificity compared with standard care would lead to increased number of referrals to memory clinics, thus having an impact on the clinic capacity. Similarly, introducing a test with increased sensitivity but reduced specificity in memory clinics as a triage tool would likely lead to more patients being routed to further testing. The costs of expanding the capacity of these services or any other services that may be impacted by an increase in diagnosed cases of dementia has not been considered in this analysis, nor has the impact of the likely increase in waiting times to access the services.
Finally, this model only considers the use of the ICA tool in place of current cognitive tests used in clinical practice. The ICA tool may have potential other uses including remote testing or monitoring of patients, although further clinical evidence and economic analyses would be needed to evaluate this.
Conclusion
Introduction of the ICA tool within the NHS as a diagnostic tool for cognitive impairment and dementia in place of cognitive assessment tools currently used in primary care could be cost saving to the NHS. The potential cost savings were even greater when the tool was evaluated as a triage tool in memory clinics; future studies are needed to collect more real-world data in memory clinic settings to investigate the generalization of the results across wider populations in such settings.
Precis
The ICA Tool is estimated to be cost-effective compared with standard cognitive assessments for dementia screening in primary care and memory clinic settings.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Approved 27/02/2020, North of Scotland Research Ethics Committee (Summerfield House, 2 Eday Road, Aberdeen, AB15 6RE, UK; +44 (0)1224 558458; nosres@nhs.net), ref.: 20/NS/0029. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
JS developed the model and wrote the manuscript. CK, MM, and S-MK-R led the study design, commented on the model, and edit/revised the manuscript. AS helped with the revisions of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by development of the model and preparation of this manuscript was funded by Alzheimer’s Research UK (ARUK) via Innovate UK. The ADePT study was funded by Innovate UK in the form of a grant to Cognetivity Ltd., in collaboration with ARUK and Sussex NHS partnership (project number: 105837).
Acknowledgments
Medical writing and editorial assistance with the preparation of this manuscript was provided by Karolina Badora, PhD. Development of the model was overseen by Michelle Green, MSc. Assistance with designing for the ADePT study protocol was provided by Dr. Naji Tabet and Panos Apostolou.
Conflict of interest
JS: funding for development of the economic analysis and manuscript was provided to York Health Economics Consortium (employer). CK: director and shareholder at Cognetivity Ltd. (developer of the ICA tool). S-MK-R: chief innovation officer and stock options holder at Cognetivity Ltd. MM: previous employee and stock options holder at Cognetivity Ltd.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2023.1240901/full#supplementary-material
Footnotes
1. ^https://pard.mhra.gov.uk/manufacturer-details/22053
2. ^https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfRL/rl.cfm?lid=755440&lpcd=PTY
References
1. Arvanitakis, Z, Shah, RC, and Bennett, DA. Diagnosis and management of dementia: review. JAMA. (2019) 322:1589–99. doi: 10.1001/jama.2019.4782
2. Alzheimer’s Association. What is dementia? Available at: https://www.alz.org/alzheimers-dementia/what-is-dementia. (Accessed February 3, 2022)
3. GBD Dementia Forecasting Collaborators. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the global burden of disease study 2019. Lancet Public Health. (2022) 7:e105–25. doi: 10.1016/S2468-2667(21)00249-8
4. Wittenberg, R, Hu, B, Barraza-Araiza, L, and Rehill, A. CPEC working paper 5. Projections of older people with dementia and costs of dementia care in the United Kingdom, 2019–2040. Available at: https://www.alzheimers.org.uk/sites/default/files/2019-11/cpec_report_november_2019.pdf. (Accessed February 4, 2022)
5. Yiannopoulou, KG, and Papageorgiou, SG. Current and future treatments in Alzheimer disease: an update. J Cent Nerv Syst Dis. (2020) 12:117957352090739. doi: 10.1177/1179573520907397
6. National Institute for Health and Care Excellence. Dementia overview. Available at: https://pathways.nice.org.uk/pathways/dementia. (Accessed February 3, 2022)
7. Farlow, M, Anand, R, Messina, J Jr, Hartman, R, and Veach, J. A 52-week study of the efficacy of rivastigmine in patients with mild to moderately severe Alzheimer’s disease. Eur Neurol. (2000) 44:236–41. doi: 10.1159/000008243
8. Rasmussen, J, and Langerman, H. Alzheimer’s disease—why we need early diagnosis. Degener Neurol Neuromuscul Dis. (2019) 9:123–30. doi: 10.2147/DNND.S228939
9. Food and Drugs Administration. FDA grants accelerated approval for Alzheimer’s drug. Available at: https://www.fda.gov/news-events/press-announcements/fda-grants-accelerated-approval-alzheimers-drug. (Accessed February 7, 2022)
10. National Institute for Health and Care Excellence. NG97: Dementia: assessment, management and support for people living with dementia and their carers. Available at: https://www.nice.org.uk/guidance/ng97/chapter/Recommendations#diagnosis. (Accessed February 4, 2022)
11. Kalafatis, C, Modarres, MH, Apostolou, P, Tabet, N, and Khaligh-Razavi, SM. The use of a computerized cognitive assessment to improve the efficiency of primary care referrals to memory services: protocol for the accelerating dementia pathway technologies (ADePT) study. JMIR Res Protoc. (2022) 11:e34475. doi: 10.2196/34475
12. Binng, D, Splonskowski, M, and Jacova, C. Distance assessment for detecting cognitive impairment in older adults: a systematic review of psychometric evidence. Dement Geriatr Cogn Disord. (2020) 49:456–70. doi: 10.1159/000511945
13. Khaligh-Razavi, S-M, Habibi, S, Sadeghi, M, Marefat, H, Khanbagi, M, Nabavi, SM, et al. Integrated cognitive assessment: speed and accuracy of visual processing as a reliable proxy to cognitive performance. Sci Rep. (2019) 9:1102. doi: 10.1038/s41598-018-37709-x
14. Khaligh-Razavi, S-M, Sadeghi, M, Khanbagi, M, Kalafatis, C, and Nabavi, SM. A self-administered, artificial intelligence (AI) platform for cognitive assessment in multiple sclerosis (MS). BMC Neurol. (2020) 20:193. doi: 10.1186/s12883-020-01736-x
15. Kalafatis, C, Modarres, MH, Apostolou, P, Marefat, H, Khanbagi, M, Karimi, H, et al. Validity and cultural generalisability of a 5-minute AI-based, computerised cognitive assessment in mild cognitive impairment and Alzheimer’s dementia. Original research. Front Psychiatry. (2021) 12:706695. doi: 10.3389/fpsyt.2021.706695
16. Karimi, H, Marefat, H, Khanbagi, M, Kalafatis, C, Modarres, MH, Vahabi, Z, et al. Temporal dynamics of animacy categorization in the brain of patients with mild cognitive impairment. PLoS One. (2022) 17:e0264058. doi: 10.1371/journal.pone.0264058
17. Naghavi, S, Ashtari, F, Adibi, I, Shaygannejad, V, Ramezani, N, Pourmohammadi, A, et al. Effect of deep gray matter atrophy on information processing speed in early relapsing-remitting multiple sclerosis. Mult Scler Relat Disord. (2023) 71:104560. doi: 10.1016/j.msard.2023.104560
18. Glassbrook, DJ, Chazot, PL, and Hind, K. Precision of the integrated cognitive assessment for the assessment of neurocognitive performance in athletes at risk of concussion. bioRxiv (2023) Available at: https://doi.org/10.1101/2023.03.22.533746. [Epub ahead of preprint]
19. Modarres, MH, Kalafatis, C, Apostolou, P, Tabet, N, and Khaligh-Razavi, SM.The use of the integrated cognitive assessment (ICA) to improve the efficiency of primary care referrals to memory Services in the Accelerating Dementia Pathway Technologies (ADePT) study. medRxiv. (2023). Available at: https://doi.org/10.1101/2023.05.31.23290793. [Epub ahead of preprint]
20. National Institute for Health and Excellence. Guide to the methods of technology appraisal. Available at: https://www.nice.org.uk/process/pmg9/chapter/foreword. (Accessed February 7, 2022)
21. NHS Digital. Recorded dementia diagnoses September 2019. (2019). Available at: https://digital.nhs.uk/data-and-information/publications/statistical/recorded-dementia-diagnoses/september-2019. (Accessed September 23, 2021)
22. NHS Digital. Recorded dementia diagnoses March 2020. (2020). Available at: https://digital.nhs.uk/data-and-information/publications/statistical/recorded-dementia-diagnoses/march-2020. (Accessed September 23, 2021)
23. Ozer, S, Noonan, K, Burke, M, Young, J, Barber, S, Forster, A, et al. The validity of the memory alteration test and the test your memory test for community-based identification of amnestic mild cognitive impairment. Alzheimers Dement. (2016) 12:987–95. doi: 10.1016/j.jalz.2016.03.014
24. Tong, T, Thokala, P, McMillan, B, Ghosh, R, and Brazier, J. Cost effectiveness of using cognitive screening tests for detecting dementia and mild cognitive impairment in primary care. Int J Geriatr Psychiatry. (2017) 32:1392–400. doi: 10.1002/gps.4626
25. Sheehan, B. Assessment scales in dementia. Ther Adv Neurol Disord. (2012) 5:349–58. doi: 10.1177/1756285612455733
26. Ciesielska, N, Sokołowski, R, Mazur, E, Podhorecka, M, Polak-Szabela, A, and Kędziora-Kornatowska, K. Is the Montreal cognitive assessment (MoCA) test better suited than the Mini-mental state examination (MMSE) in mild cognitive impairment (MCI) detection among people aged over 60? meta-analysis. Psychiatr Pol. (2016) 50:1039–52. doi: 10.12740/PP/45368
27. Tsoi, KK, Chan, JY, Hirai, HW, Wong, SY, and Kwok, TC. Cognitive tests to detect dementia: a systematic review and meta-analysis. JAMA Intern Med. (2015) 175:1450–8. doi: 10.1001/jamainternmed.2015.2152
28. Aldus, CF, Arthur, A, Dennington-Price, A, Millac, P, Richmond, P, Dening, T, et al. Undiagnosed dementia in primary care: a record linkage study. NIHR journals. Library. (2020) 8:1–108. doi: 10.3310/hsdr08200
29. Teipel, SJ, Ewers, M, Reisig, V, Schweikert, B, Hampel, H, and Happich, M. Long-term cost-effectiveness of donepezil for the treatment of Alzheimer’s disease. Eur Arch Psychiatry Clin Neurosci. (2007) 257:330–6. doi: 10.1007/s00406-007-0727-1
30. Bradford, A, Kunik, ME, Schulz, P, Williams, SP, and Singh, H. Missed and delayed diagnosis of dementia in primary care: prevalence and contributing factors. Alzheimer Dis Assoc Disord. (2009) 23:306–14. doi: 10.1097/WAD.0b013e3181a6bebc
31. Kind, P, Hardman, G, and Macran, S. UK population norms for EQ-5D No 172chedp, Working Papers Centre for Health Economics, University of York (1999) Available at: https://EconPapers.repec.org/RePEc:chy:respap:172chedp.
32. Handels, RLH, Wimo, A, Dodel, R, Kramberger, MG, Visser, PJ, Molinuevo, JL, et al. Cost-utility of using Alzheimer’s disease biomarkers in cerebrospinal fluid to predict progression from mild cognitive impairment to dementia. J Alzheimers Dis. (2017) 60:1477–87. doi: 10.3233/JAD-170324
33. Orgeta, V, Edwards, RT, Hounsome, B, Orrell, M, and Woods, B. The use of the EQ-5D as a measure of health-related quality of life in people with dementia and their carers. Qual Life Res. (2015) 24:315–24. doi: 10.1007/s11136-014-0770-0
34. Wimo, A, Reed, CC, Dodel, R, Belger, M, Jones, RW, Happich, M, et al. The GERAS study: a prospective observational study of costs and resource use in community dwellers with Alzheimer’s disease in three European countries-study design and baseline findings. J Alzheimers Dis. (2013) 36:385–99. doi: 10.3233/JAD-122392
35. Gomes, M, Pennington, M, Wittenberg, R, Knapp, M, Black, N, and Smith, S. Cost-effectiveness of memory assessment services for the diagnosis and early support of patients with dementia in England. J Health Serv Res Policy. (2017) 22:226–35. doi: 10.1177/1355819617714816
36. PSSRU. Unit costs of health and social care. Available at: https://www.pssru.ac.uk/project-pages/unit-costs/
37. Yokomizo, JE, Seeher, K, de Oliveira, GM, Zanesco, L, Andrade-Silva, FB, Leonhardt, MC, et al. Cognitive screening test in primary care: cut points for low education. Rev Saude Publica. (2018) 52:88–8. doi: 10.11606/S1518-8787.2018052000462
38. Tumas, V, Borges, V, Ballalai-Ferraz, H, Zabetian, CP, Mata, IF, Brito, MMC, et al. Some aspects of the validity of the Montreal cognitive assessment (MoCA) for evaluating cognitive impairment in Brazilian patients with Parkinson’s disease. Dement Neuropsychol. (2016) 10:333–8. doi: 10.1590/s1980-5764-2016dn1004013
39. Cordell, CB, et al. Alzheimer’s & dementia (2013) 141–150. Available at: (Table 3) https://www.alz.org/getmedia/9687d51e-641a-43a1-a96b-b29eb00e72bb/cognitive-assessment-toolkit
40. Centre for Economics and Business Research (CEBR). The economic cost of dementia to English businesses—2019 update. A report for Alzheimer’s Society. (2019). Available at: https://www.alzheimers.org.uk/sites/default/files/2019-09/The%20economic%20cost%20of%20dementia%20to%20English%20businesses%20-%20edited.pdf. (Accessed September 30, 2021)
41. Getsios, D, Blume, S, Ishak, KJ, Maclaine, G, and Hernández, L. An economic evaluation of early assessment for Alzheimer’s disease in the United Kingdom. Alzheimers Dement. (2012) 8:22–30. doi: 10.1016/j.jalz.2010.07.001
42. Anderson, R, Knapp, M, Wittenberg, R, Handels, R, and Schott, J. Economic modelling of disease-modifying therapies in Alzheimer’s disease. London School of Economics Personal Social Services Research Unit (2018). Available at: https://www.lse.ac.uk/cpec/assets/documents/EconomicmodellingAD.pdf
43. Cook, L, Souris, H, and Isaavs, J. London memory services 2019 report. (2019). Available at: https://www.england.nhs.uk/london/wp-content/uploads/sites/8/2019/11/FINAL-London-memory-service-audit-2019.pdf. (Accessed September 23, 2021)
44. Beishon, LC, Batterham, AP, Quinn, TJ, Nelson, CP, Panerai, RB, Robinson, T, et al. Addenbrooke’s cognitive examination III (ACE-III) and mini-ACE for the detection of dementia and mild cognitive impairment. Cochrane Database Syst Rev. (2019) 2019:CD013282. doi: 10.1002/14651858.CD013282.pub2
45. Molloy, DW, and Clarnette, R. Standardized Mini-mental state examination (SMMSE) a user’s guide. Available at: https://www.swlstg.nhs.uk/images/Standardised_mini-mental_state_examination.pdf. (Accessed February 17, 2022).
46. Dubois, B, Padovani, A, Scheltens, P, Rossi, A, and Dell’Agnello, G. Timely diagnosis for Alzheimer’s disease: a literature review on benefits and challenges. J Alzheimers Dis. (2016) 49:617–31. doi: 10.3233/JAD-150692
47. Banerjee, S, and Wittenberg, R. Clinical and cost effectiveness of services for early diagnosis and intervention in dementia. Int J Geriatr Psychiatry. (2009) 24:748–54. doi: 10.1002/gps.2191
48. British National Formulary (BNF). Available at: https://bnf.nice.org.uk/. (Accessed September 30, 2021)
49. Department of Health Dementia Policy Team. Prime Minister’s challenge on dementia (2020). Available at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/507981/PM_Dementia-main_acc.pdf. (Accessed March 23, 2022).
50. Kantarci, K. 2021 marks a new era for Alzheimer’s therapeutics. Lancet Neurol. (2022) 21:3–4. doi: 10.1016/s1474-4422(21)00412-9
51. Lam, J, Hlávka, J, and Mattke, S. The potential emergence of disease-modifying treatments for Alzheimer disease: the role of primary care in managing the patient journey. J Am Board Fam Med. (2019) 32:931–40. doi: 10.3122/jabfm.2019.06.180328
52. Barnett, JH, Lewis, L, Blackwell, AD, and Taylor, M. Early intervention in Alzheimer’s disease: a health economic study of the effects of diagnostic timing. BMC Neurol. (2014) 14:101. doi: 10.1186/1471-2377-14-101
53. Dixon, J, Ferdinand, M, D’Amico, F, and Knapp, M. Exploring the cost-effectiveness of a one-off screen for dementia (for people aged 75 years in England and Wales). Int J Geriatr Psychiatry. (2015) 30:446–52. doi: 10.1002/gps.4158
54. Prince, M, Knapp, M, Guerchet, M, McCrone, P, Prina, M, Comas-Herrera, A, et al. Dementia UK update: Second Edition - Overview. Alzheimer’s Society. (2014). Available at: https://kclpure.kcl.ac.uk/portal/en/publications/dementia-uk-second-edition-overview
Keywords: ICA, CognICA, dementia, cognitive screening, AI, National Health Service (NHS), health economics
Citation: Shore J, Kalafatis C, Stainthorpe A, Modarres MH and Khaligh-Razavi S-M (2023) Health economic analysis of the integrated cognitive assessment tool to aid dementia diagnosis in the United Kingdom. Front. Public Health. 11:1240901. doi: 10.3389/fpubh.2023.1240901
Edited by:
Ismaeel Yunusa, University of South Carolina, United StatesReviewed by:
Christos Theleritis, National and Kapodistrian University of Athens, GreeceAmey Rane, Agios Pharmaceuticals (United States), United States
Copyright © 2023 Shore, Kalafatis, Stainthorpe, Modarres and Khaligh-Razavi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Seyed-Mahdi Khaligh-Razavi, Seyed@Cognetivity.com
 Judith Shore1
Judith Shore1