Discover ACP-01:
Transforming Lives With Autologous Stem Cell Therapies
Groundbreaking Science.
New Treatments.
New Hope.
At Hemostemix, we are developing safe and effective autologous stem cell platforms that create new options, treatment protocols and hope for millions of patients globally.
Our proprietary ACP-01 autologous (patient’s own) therapy, has been extensively studied in multiple clinical trials for Angina, Ischemic and Dilated Cardiomyopathy, Peripheral Arterial Disease (PAD) and Chronic Limb Threatening Ischemia (CLI).
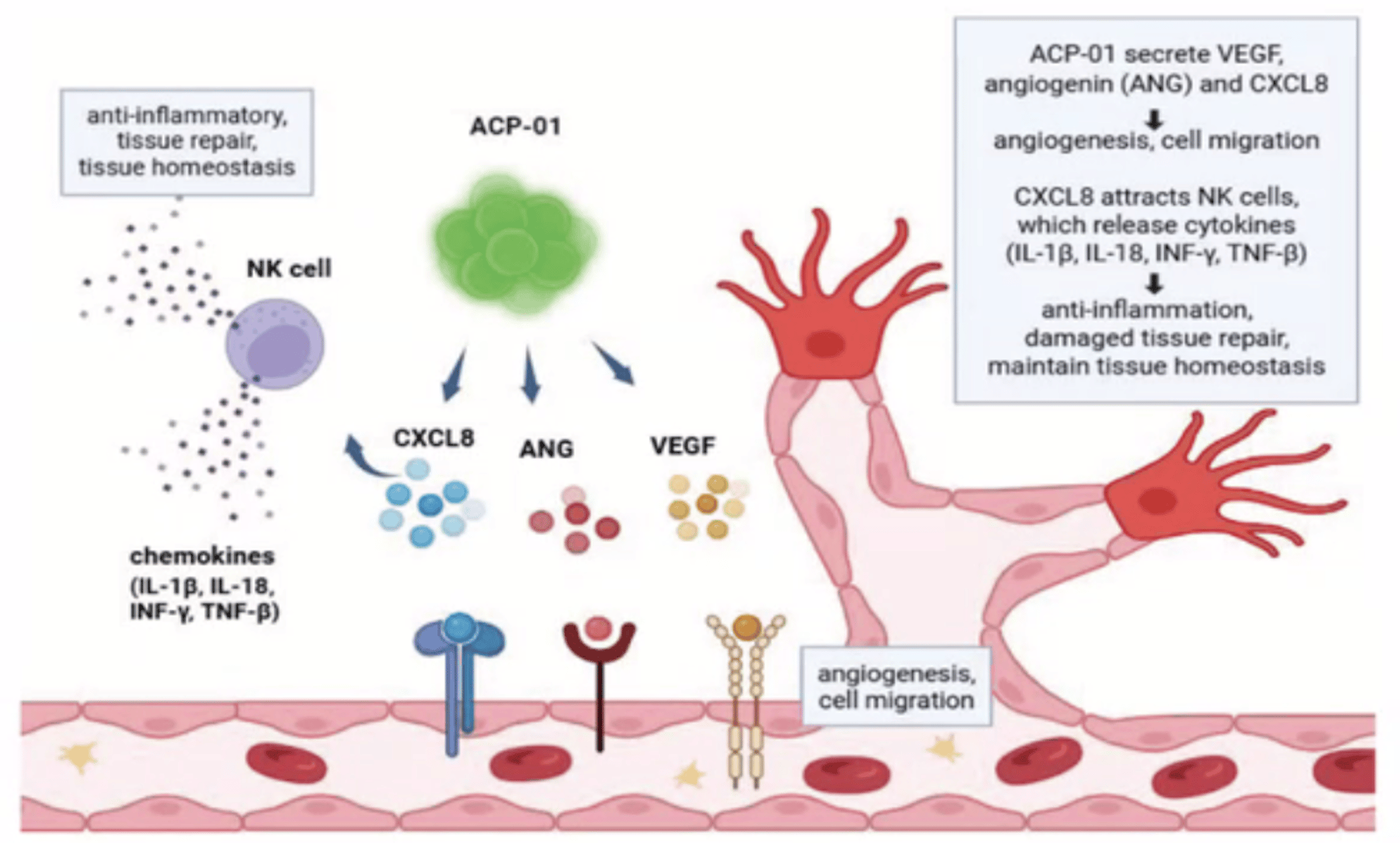
How ACP-01 Works
ACP-01 Helps Generate New Blood Vessels and Restore the Flow of Oxygen and Nutrients To Areas Damaged By Ischemia
- FIND: ACP-01 migrates to areas of decreased blood flow (Ischemia)
- EMBED: ACP-01 embeds into injured tissues and repopulates injured tissue
- RELEASE: ACP--01 releases growth factors and reduces inflammation
- GENERATE: ACP-01 generates new blood vessels to improve blood flow
Autologous Technology Delivers Significant Benefits
No Ethical Conflicts
Unlike fetal stem cells, autologous cells are collected from the patient's own body.
Low Patient Burden
- Collection is via standard blood draw
- No anesthesia
- Avoids bone marrow harvest complications
- Implant is via local injection
Risk Reduction
- No tainted blood or blood products
- Avoids immune rejection and graft-versus host disease (GVHD)
- No risk of neoplasia (abnormal, new, uncontrolled cell growth) from pluripotent cells (cells that can develop into many different types of cells or tissue)
Research Spotlight
Read this informative study demonstrating the efficacy and safety of ACP-O1 in select patients with no option Critical Limb Ischemia. Key points highlighted in this paper include :
- ACP-01 processing
- Ulcer response to treatment
- Survival and amputation rates
- Visual analog pain scores
Meet Our Patients
Disclaimer
-
ACP-01 is an investigational cell therapy. It has not been approved by the U.S. Food and Drug Administration (FDA) or any other regulatory authority for commercial sale.
-
The safety and effectiveness of this therapy have not been established. Information on this website is for educational purposes only and should not be considered medical advice or a promotion of an unapproved product.
-
Access to this investigational therapy is available in Florida pursuant to state law (s. 381.985, F.S.) for eligible patients who have provided informed consent and have been advised of the investigational nature of the treatment.
Information
Angiogenic Precursor Cell Treatment of Critical Limb Ischemia Decreases Ulcer Size, Amputation and Death Rate: Re-Examination of phase II ACP NO-CLI Trial Data
Fraser C Henderson*, Ina Sarel, Kelly Tuchman, Stephen Lewis and York Hsiang
Volume5-Issue2
Dates: Received: 2024-01-18 | Accepted: 2024-02-01 | Published: 2024-02-02
Pages: 092-105
Abstract
Introduction: Critical limb ischemia has a prevalence in the US of 1.33%, with mortality 15-20% and major amputation 10-40% per year. Stem cell treatment has emerged as a treatment option for the 45% of patients for whom revascularization procedures are not possible.
Objective: This study re-examines the data of the Phase II clinical treatment of no option Critical limb ischemia with Hemostemix’ angiogenic cell precursors, focusing upon ulcer wound healing, amputation and death rate of this cohort.
Methods: Primary endpoints were changes in ulcer size and major amputation or death within one year of treatment. The secondary endpoint was change in pain level.
Results: From 2015 to 2021, 67 patients with no option Critical limb ischemia were allocated to treatment with ACP-01 (46/67) or placebo (21/67). From this data, only patients who presented with wound ulcers before administration of ACP-01 were reviewed (21 treatment, 8 placebo). Ulcer size in the treated group decreased from a mean of 1.46 cm2 to 0.48 mm2 (p = 0.01) by 3 months. There was no significant decrease in the size of the ulcers of the placebo group (p < 0.54). At one year there were no complications related to treatment. The treatment group had one amputation (4.8%) and one death (4.8%); the placebo group had 2 amputations (25%) and 1 death (12.5%). Change in pain was not significant in either group at 3 months, but at 1 year was improved in the placebo group (p = 0.01).
Conclusion: The administration of ACP-01 within a program of careful patient follow up is safe and associated with reduced ulcer size and decreased rate of amputation and death. Consideration should be given to re-administration of stem cell treatments every 3-6 months to optimize improvement of Critical limb ischemia. Further studies, more appropriately powered, are warranted.


